Chemical Education Journal (CEJ),
Vol. 10, No. 2 /Registration No. 10-15/Received May 13, 2008.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
Most universities around the world accept that all of their graduates
need to develop a range of skills, not only in their specific
discipline, but more generic 'transferable skills' too. In the
UK, several drivers have ensured that this is the case for chemistry:
in 1997, the Dearing report recommended that all degrees should
empower graduates with so-called 'key skills; several reports
from chemical industries identified team work, report-writing,
time-management, and presentation skills as essential characteristics
of their employees; finally, the Quality Assurance Agency required
all subjects to specify programme outcomes for all degrees, and
a range of transferable skills are now embedded in the descriptors
for all degrees, including chemistry.
If transferable skills are both required and desirable in our graduates, what is the best way of delivering them within our degree programmes? One approach would be to teach them generically using a central university facility - this has many obvious advantages such as the efficient use of resources, and the employment of lecturers who have the specialist expertise. I will argue, however, that the students only really engage with this important part of their education if it is embedded within their degree, involves the use of their chosen subject in various 'transferable skills' activities, and is delivered by their chemistry lecturers.
This paper is based on a presentation to the 12th Asian Chemical Congress (Kuala Lumpur, Malaysia, 2007); in it, I will explain the background to the importance of transferable skills in chemistry degree programmes in the UK, provide some guidance for developing appropriate exercises, and offer further details for two examples of such exercises. The material is based on a 'Communicating Chemistry' module developed at Heriot-Watt University (Bailey, 1997, 2001; Bailey & Shinton, 1999; Fry et al., 2003), and more specific exercises that have been developed subsequently (e.g. Bailey, 2005, 2007).
Introduction: what skills should my graduates have?
Three major factors have led to the acceptance in the UK that graduates should not only possess their subject-specific skills, but also that they need to have transferable skills.
a) The Dearing Report. The Dearing committee was composed of individuals from a diverse range of backgrounds: academic, industrial, educational, public sector (Dearing, 1997). They appeared to be strongly united in their support for the final report, which made 93 main recommendations. Of these, three have had a major impact on the universities in the UK. The first concerned student fees, which were intended to provide money to help redress the serious under-funding of the infrastructure of universities. The second concerned the provision of wider access to HE. The third concerned the content of HE degrees, and what they should aim to provide. The Dearing Report:
"emphasised the need for students and employers to be well-informed about what higher education offers. They need clear statements about the intended outcomes of higher education programmes..."
Moreover, the report (paragraph 38) stated that:
"There is much evidence of support for the further development of a range of skills during higher education, including what we term the key skills of communication, both oral and written, numeracy, the use of communications and information technology and learning how to learn. We see these as necessary outcomes of all higher education programmes."
Underpinning this is recommendation 21, which requires all degrees to have a 'programme specification', which "gives the intended outcomes of the programme in terms of:"
i. Knowledge/understanding of subject (syllabus)
i. Special subject skills (e.g. lab work)
iii. Cognitive skills (methodology, critical analysis)
iv. Key skills
Traditionally, chemistry degree programmes covered aspects i-iii of the 'programme specification', but 'key skills' (especially 'communication') have been a minor feature of degrees, or were not explicitly covered at all.
b) Quality Assurance Agency. Although teaching audits had been underway for some years, the Dearing Report certainly influenced the way that the QAA modified its assessment procedures subsequently. In particular, Chemistry, History and Law underwent trial assessments in 1998, in which the programme specification was a major feature. In order to provide some sort of national framework for the core requirements of degrees in particular subjects, the benchmark documents were produced by appropriate bodies; for Chemistry, this was the Royal Society of Chemistry (Quality Assurance Agency, 1998). Their programme specification for chemistry mirrored Dearing's, with 4 main headings as summarised below:
Programme specification - Chemistry benchmark:
i. Subject knowledge (syllabus)
ii. Chemistry related cognitive abilities and skills
iii. Chemistry related practical skills
iv. Transferable skills
'Transferable skills' were largely equivalent to Dearing's 'key skills', and had the following sub-headings:
Since then, accredited chemistry degrees have had to address the issue of transferable skills, and these remain an essential component of degrees, and the key skills have remained largely unaltered in subsequent revisions of the chemistry benchmark (Quality Assurance Agency, 2007).
c) Employers. In 1999, Duckett, Garratt and Lowe published the results of an extensive survey of recent chemistry graduates (Duckett et al., 1999). The types of employment were wide-ranging, and about a quarter were in non-chemistry jobs (e.g. financial management, civil service, business administration). Summarising the results of the survey, the following seven areas are the ones that were identified as very important, and for which graduates felt that their university training had been inadequate (roughly in this order):
Notable is that the chemistry syllabus does not feature (i.e. graduates appeared to have the necessary 'chemistry' skills) - and yet this is probably the issue that is debated most heatedly in chemistry departments. All of the concerns can be considered as aspects of transferable skills, and most could be regarded as communication skills. In the same paper, Duckett et al. reported the following top seven areas of deficiency of recent graduates, as perceived by chemical company employers:
This view of recent graduates was reinforced in The Mason Report (1998) (Mason, 1998); of major issues identified by exployers, "...concern was expressed about weakness in interpersonal and communication skills, accuracy in documentation and practical laboratory skills."
So the findings from a major government report, the Quality Assurance Agency, and the chemical industry all agree that chemistry graduates must possess a range of key transferable skills, as well as the subject-specific abilities that one would expect of a graduate chemist. But who should teach these skills to our students?
Who should teach transferable skills to our students?
There is a strong case for teaching communication skills centrally, and some arguments that have been put forward in support of this include the following:
Although I have reservations about most of these statements, they provide a valid argument for the centralised teaching of generic communication skills at universities. But my own experience of students is that they will disengage from activities that seem irrelevant to them, and such centralised teaching appears to be so for three reasons:
1) Students believe that the skills needed to communicate effectively in chemistry are subject-specific. Many of our students feel that they have come to university to study chemistry, and that is what we should teach them - they simply will not accept that being taught generic transferable skills is relevant to them, and to the subject they have chosen.
2) It is very hard to build in a marks scheme that gives appropriate weighting to generic communication skills. If many marks are assigned to it, then departments argue that the subject-specific degree is undervalued; but if the weighting is low, students perceive it as being regarded as low priority, and are likely to be content with a modest performance (e.g. getting minimum pass mark if it is a course requirement).
3) We must not forget that many of our students regard us as role models. If we are seen to regard transferable skills as important by teaching it ourselves, within a chemistry context, then students realize how important we think it is. Conversely, if we say that the skills are important, but we are not actually involved in teaching them, then the students are likely to feel that we do not really value this aspect of their education.
So, despite the strong case for the centralised tuition of communication skills, I think that these skills must be taught within a chemistry context, so that the students understand their relevance.
How should we teach transferable skills?
Although there is a case for teaching key skills centrally, it is my firmly held view that they should be part of the teaching and learning of chemistry degrees within our departments; the reason for this is simply relevance. There are three other important aspects of communication skills:
In theory, the skills are probably best taught in exercises that are entirely embedded in the chemistry degree programme, but this requires excellent cooperation from all colleagues delivering the chemistry degree programme, and careful monitoring of the skills when programme revisions take place. Many universities in the UK (e.g. Heriot-Watt University in Edinburgh, and the University of Manchester) have specific modules that focus on transferable skills within a chemistry context; this provides foundations on which the skills can be developed later on in the programme (e.g. in final year projects), and also ensures that students must participate fully in order to pass the module. From a quality assurance perspective, this approach also makes it easier to define and prove that the key skills are developed within the degree. When this was first developed at Heriot-Watt University, we used 10 exercises during an 8-week term, in a module that required about 100 hours of work from the students:
Communicating Chemistry
| Week 1 | Fluorofen problem (industrial, team exercise) |
| Scientific paper (comprehension) | |
| Week 2 | Keyboard skills (using software to prepare material) |
| New Chemist assignment (writing a short article) | |
| Week 3 | Information retrieval (Chemical Abstracts and Web of Science) |
| Week 4 | Dictionary of Interesting Chemistry |
| Week 5 | Chubli Fruit project (multi-part team exercise) |
| Week 6 | Annual review (individual oral presentations) |
| Week 7 | Interviews (they attend and conduct interviews) |
| Week 8 | Team project (research plan, presented as a poster) |
The exercises cover aspects of information retrieval, comprehension, report-writing, poster preparation, oral presentation, problem-solving, critical thinking, time management, team work, and a range of interpersonal skills. More information on the module can be found elsewhere (Bailey 1997; Bailey & Shinton 1999), and some of the features that help such a module to run successfully were:
Three of these points are worthy of elaboration:
i) Setting the scene and requiring tight deadlines is important. If students are asked to carry out some background reading, not all of them will do so. However, there is an excitement and involvement from being suddenly required to tackle an urgent problem, which is simply lost if material is distributed beforehand, and this can be achieved by setting a plausible scenario from cold (e.g. an urgent problem that a manufacturing company must solve; an article that must reach an editor by a deadline; a presentation that has been requested at short notice). The scenario can be set using a role play, a short explanation, or simply by handing out an 'urgent memo'.
ii) Peer pressure is a huge incentive, and requiring their work and presentations to be seen (and hence judged) by their peers is one of the strongest incentives for students to produce high quality work. However, students are often appalling at marking work. To state an obvious problem, a weak student will often regard a poor piece of work as quite good, whereas very able students are usually harsh in their marking. One useful compromise is to discuss and agree a marking scheme with the class for some of the exercises. However, students are good at perceiving high quality work, and there is always strong agreement between students about the best pieces of work from an exercise, which usually matches the tutor's views. Students may be poor at marking isolated pieces of work, but they can recognize quality when they see it, and the peer selection process can usefully be used in the allocation of modest prizes. Using peer judgement also has the added advantage that some students might not be clear what was wrong with their piece of C-rated work, but they can clearly see that someone else's was worth an A grade.
iii) Feedback and assessment are essential components of any programme that aims to develop communication skills (Bailey, 1999). Whilst peer pressure has a massive effect on encouraging high quality work, we also require students to pass every component of our 'Communicating Chemistry' module; they cannot get away without having had a valid attempt at everything, and everything they attempt is given a letter grade. They also get extensive feedback, although just as important is providing them with copies of the best work from their colleagues, so that they can see high quality examples. Their feedback can be collated into a final feedback sheet, and it is worthwhile to require them to use this to help them produce a summary of their strengths and weaknesses.
Two examples of transferable skills exercises
Below are two examples of exercises that I have designed as an introduction to problem solving and team work. They exemplify the style of the learning involved in many of the 'transferable skills' exercises, in which a scenario is set up in which they need solve a problem and/or deliver an output; the emphasis is on the students developing the skills they need, rather than the tutor attempting to teach them the skills.
1) The 'Poor Raymond' exercise (Bailey, 2005, 2007)
In summary, the scene is set that my brother has died under mysterious circumstances, although the police thought that his death was accidental. I have managed to obtain three pieces of forensic material, and require my 'chemistry student friends' to carry out some analyses of them, to see whether we can find sufficient evidence for the police to launch a formal enquiry. The timescale is very tight, so the students must decide what needs doing, divide the tasks amongst themselves, and deliver an oral and written report within a week.
This is an exercise that I developed at the University of Manchester, and was delivered to first year students (total 200) in the middle of their 1st semester. I delivered it on my own, to a cohort of around 100 students; a re-run a few weeks later used different compound mixtures (see details below), to preclude copying.
The main aims of the exercise were threefold:
a) To let the students have the fun of solving a (ficticious) problem that required them to use their limited chemistry knowledge - the 'fun' part was really important, as we can sometimes get bogged down with ensuring that we deliver the chemistry content, whilst forgetting that we need to inspire our students.
b) To get them working in teams, and developing the associated interpersonal skills.
c) To give them practice at problem-solving - i.e. devising their own way to come up with an answer to a problem, even if their knowledge is limited and the experiments will be imperfect.
They of course develop other skills (e.g lab work, report-writing, time-management), but one has to be realistic - one exercise requiring only about 10h work can only have a limited impact, so I regard these as peripheral benefits.
Structure of the exercise
The exercise was run over a 1 week period as follows:
| a) Introduction to the exercise | Thursday | 1h lecture slot |
| b) Conduct experiments | Monday | 3h lab period |
| Tuesday | 3h lab period | |
| c) Debriefing | Thursday | 1h lecture slot |
Including discussion and write-up (requiring about 2h), we allocated about 10h of student study time to the entire exercise.
What did the students do?
a) Introduction to the exercise (1h lecture slot): I carried this
out as a role play, with the scene being set, and the exercise
acted out, by myself as 'the brother of poor Raymond'. The students
arrived with no prior information about the exercise, and no hand-outs
were given until the scene was set. This tactic helped to engage
the students, who were then divided up into groups of about 6
- one can either reinforce tutorial groups, or help the students
to mix by assembling the groups appropriately. The introduction
takes about 20 minutes, at which point they are given the handouts
needed to carry out the exercise (see below for the handouts they
receive); the students are then free to plan the experiments as
they think best, and/or to agree to meet up later to plan them.
b) Conduct experiments (6h of lab time): the experiments are designed to be too lengthy to be all carried out sequentially within the two 3-hour lab sessions unless the tasks are divided up amongst the group; however, they are short enough that most of the analyses can be carried out in the first lab day, so that they can (often as a group) return to some aspect of the experiments that seemed ambiguous, or for which they would like to obtain additional data. They are asked to prepare a one-page team report for the police from all of their analyses, and also decide what steps they might take to preserve the integrity of material that might be re-analysed professionally.
c) Debriefing (1h workshop slot): they must not only submit their report, but also be ready to give their results to the whole class - not actually too demanding, but it gives them some practice at sharing results with a bigger group. This feedback session is very important, as the students start to make some judgment of the validity/reliability of their results. We have run it with around 15 groups, and so can select 5 groups to provide their results on each of the 3 experiments, each time checking with the rest of the students to see how much agreement there is. This is effectively the same as repeating the results as an analytical scientist would, although the variation isn't analysed statistically. A key aspect of the debriefing is when the students ask "what is the right answer?" Although there effectively is a correct solution, in that the samples were prepared artificially, a critical aspect of the exercise is that the 'correct' answers are the results the students obtain - to act out the role-playing realistically, there are no 'right answers', just a balance of scientific evidence that leads to a proposal - quite a revelation for many first years!
What handouts do the students get?
As indicated above, they receive no handouts before the exercise,
or at the start of it. After the introduction has been acted out,
I hand out a summary of this to each student (handout 1), and
one copy of some additional information is given to each group.
I then leave the lecture theatre! This has quite a dramatic effect
- usually a minute or so or rather stunned silence, then a realisation
by each group that they have all the information that they're
going to get, and it is now up to them as a group to plan what
they will do, and do it!
Poor Raymond (handout 1)
The death of my brother Raymond was a great shock to me, and to all his friends and family. At 20 years old, keen on all racket and team sports, he was found dead at his place of work, MacKenzies Winery, just 3 days ago. Although the inquest has yet to be held, preliminary enquires by the police suggest 'death by misadventure'. He had been taking a mixture of pain-killers and anti-inflammatory tablets (aspirin and ibuprofen tablets found in his pockets) to help him play squash through a shoulder injury, and an adverse reaction with alcohol at a work's celebration apparently led to a fatal reaction. Raymond had already raised concerns with me about the practices of his present employer, for whom he'd worked for a couple of years. Although leaving school at 16 and going straight into a job, Raymond was a bright guy, and the 'Research and Development Assistant' role he took on was little more than a general skivvy. And the firm didn't seem keen or supportive when he wanted to understand about the underlying science, or when he suggested that he might try to obtain a University degree in chemistry after taking A-levels in evening classes.
Yesterday, one of his close colleagues came to see me. Michael Fletcher had worked alongside Raymond, and the two had discussed their concerns about the additives that the company was using to improve the wines they were selling.
To increase the value of the wine, the company was illegally adding flavourings to the vats. Raymond had managed to smuggle some of this liquid into a vial, which Michael had found in Raymond's locker; they suspected that two components were present, which made the wine sweeter and smell more fruity.
In the step to help remove the insolubles from the wine-making process, the company was meant to add innocuous tartaric acid, but Raymond suspected that a more efficient but illegal alternative was being used. Some of this had splashed onto his lab-coat, and Raymond had cut this out, hoping to isolate and identify the additive, thought to be an aromatic acid.
Finally, Michael had retrieved an almost empty unlabelled bottle from the lab bins. It contained a small amount of a white powder. Michael wondered if one of the firm had been worried that Raymond was finding out too much, and had decided to lace his lunch with drugs, hoping he might then fall asleep on the job and so give the firm an excuse to sack him. If true, this had had unexpected and fatal consequences.
The police suspect nothing, so I need to obtain proof of foul play. I'm hoping you can identify the wine additives from the samples collected by Raymond. Also, if the powder in the bottle were to turn out to have the same mixture of analgesics that Raymond had in his bloodstream (path lab results still pending), then this would be an extraordinary coincidence that the police would have to follow up. As trained chemists, you need to:
| a. | Identify the two components in the liquid additive. |
| b. | Identify the solid on the lab coat. |
| c. | Gain evidence for the composition of the powder, remembering that ibuprofen and aspirin packets were found on Raymond. |
| d. | Think about procedures you would carry out to try to ensure that forensic data obtained from the bottle would subsequently stand up in court. |
Remember, we're not in a position to carry out a rigorous forensic analysis. What we do want to do is to see if we think Raymond's death was suspicious, and obtain enough evidence to persuade the police to carry out further investigations. In teams of 5-7, you need to plan what you will do, check your method with a colleague (the demonstrators are suitable experts), carry out the analyses next Monday/Tuesday, and report the results at a meeting on Thursday.
Poor Raymond (handout 2)

The tips of course provide very strong guidance as to how they might carry out the analyses, and suitable chemicals (including packets of the painkillers from a pharmacist) are available in the lab session. At the bottom of the second handout is a more specific statement of what they need to do, and the deadlines they need to meet:
At the debriefing workshop at 3.00 p.m. on Thursday of week 7: you will need to have completed a brief ONE-PAGE report (free-hand is fine, but the one page limit mustn't be exceeded), to hand in to the police. You need to explain what you did, your results, your conclusions, and what each member of your team did for the report. It needs to be clear and succinct, as the police will need to discuss your report with their experts. So that we can collate results at the debriefing on Thursday, you need to bring these results with you, and you will be asked to enter some of them in a grid. You will need to be able to comment on the confidence you have in the results (or why you are not very confident), and you should also be able to suggest how your results might be 'defended' in court.
Feedback and observations
The student feedback for the exercise was excellent - they really
enjoyed tackling a 'real' problem, even they knew that it was
completely artificial. As indicated earlier, it is quite a novel
concept to them that there isn't a 'correct' answer, and pooling
their results in the debriefing session introduces the ideas of
multiple runs and confidence limits. We found this exercise ran
well with year 1 students, who could use their limited knowledge
to good effect; one could clearly adapt this sort of exercise
to more experienced students by having more complex compounds,
and requiring more challenging purification and analysis (for
example, using NMR and other spectroscopic techniques).
2) The Fluorofen problem (Bailey, 1997)
For this exercise, the students are presented with a problem concerning the economic production of an important drug that is coming out of patent. They are asked to come up with a range of ways of addressing the fact that a competitor is planning on selling 'your' blockbuster drug Fluorofen for 30% less than you intend to, when it comes off patent. This could have serious consequences for your company, and a group of chemists (i.e. your students, role-playing as company employees) are brought together to come up with some smart solutions to the problem.
I have run this exercise for years 1 and 3 of our BSc/MChem programmes. For year 1, the main aims are to foster team working and problem-solving. For the year 3 exercise, more demanding chemistry is required, as well as retrieving information from the literature and report-writing.
Structure of the exercise
The exercise can be run as a 1 hour workshop (e.g. for year 1
students), or as a more extensive exercise (e.g. 2 workshops,
6h of private study, and possibly associated laboratory experiments).
What did the students do?
This exercise was run entirely in role-play, with myself acting
as a Head of Medicinal Chemistry for a big pharmaceutical company;
the students are 'employees', brought in at short-notice to brain-storm
the problem that has arisen. The students need to work in teams
to come up with ideas for reducing the cost of manufacturing Fluorofen;
in the 3rd year exercise, they then need to analyze the formation
of a major side-product, and try to find a way of reducing its
formation, thereby increasing the yield of Fluorofen.
What handouts do the students get?
As with Poor Raymond, they receive no handouts before the exercise,
or at the start of it. I then present the scenario using PowerPoint,
and they receive copies of the appropriate slides (actually slightly
modified, so there is more detail on the handouts). The following
section explains how the exercise unfolds.
Running the Fluorofen exercise
a) Slide 1. The introduction just explains who I am (Head
of Medicinal Chemistry at ACE Pharmaceuticals), the importance
of Fluorofen medicinally and for the company, and the problem
of the competitor undercutting our post-patent price:

b) Slide 2. The second slide shows our current synthesis
of Fluorofen, and asks the teams to come up with suggestions for
reducing the cost of production (or explaining why Zenaxo can
undercut us). They have about 20-30 minutes to discuss this, and
I'll then pool ideas.
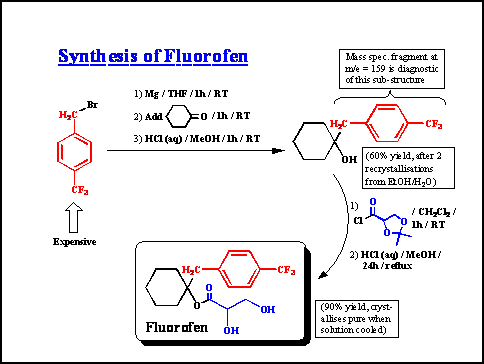
The great thing about this exercise is that there are dozens of sensible suggestions, although only a few of them will probably work. Here are 10 possibilities:
All of these are reasonable ideas, even if some of them (e.g. 'use an analogue of Fluorofen') are impractical. However, even if only half of the ideas lead to a saving of 2-3% each, this will make a big impact on the production costs. However, the really big issue concerns the reasons for the low yield in step 1, which leads on to the question:
'Why do the 3 stages of step 1 only go in 60% yield?'
This might be a suitable place to finish the exercise with 1st years but, once this has been identified as critical, you can reveal that a major by-product has been isolated, for which some structural information is available. With a bit of guidance, even 1st years can identify the dimeric nature of the by-product from its mass spectrum (see slide 3):
c) Slide 3. The next question is 'what is the structure
of the by-product D?' The mass spectrum is really enough to identify
it (so leave out the NMR for 1st years), and some simple logic
reveals D as 4,4'-(F3C-C6H4-C6H4-CF3).

d) Slide 4. The last slide is really for more advanced
students, and requires them to deduce how D might have been formed.
Once this has been achieved, one can infer how to reduce its formation
(keep the concentration of the starting bromide low, or raise
the concentration of magnesium). A couple of excellent papers
guide one to a way of increasing the effective magnesium concentration;
my final slide doesn't usually include the paragraph at the bottom,
but this could be given to 1st years, thereby providing a satisfactory
'solution' to the exercise. For more advanced students, they might
be expected to deduce the mechanism, find the possible solution
from the literature (with some clues), possibly carry out some
lab work, and then produce a final report.

Feedback and observations
Again, the feedback from this exercise has been excellent, with
students citing 'team work 'and 'problem-solving' as the best
features of the exercise (in line with the main learning outcomes
that we'd hoped to develop). In my opinion, they enjoy the exercise
not only because they are learning in a new and interactive way,
but also because they feel that they are genuinely contributing
to a 'problem' within their discipline. It is worth noting, however,
that these sorts of exercises need a lecturer who (ideally) is
happy to act out a role; the tutor must also be able to run plenary
sessions in which wide-ranging suggestions are provided by the
students, and they need to have the ability to steer the discussion
forward within the time available. This isn't quite as difficult
as it may sound, but it does require an academic with confidence.
However, it is often a revelation that the students really do
have some very good ideas, and so these exercises can be stimulating
both for the students and for the lecturer.
In my opinion, transferable skills are an essential part of any chemistry degree programme. The key conclusions that I draw from my own involvement in this, based on an extensive analysis of feedback from students (Bailey, 1997), are as follows:
a) the skills must be embedded in the chemistry programme;
b) a wide range of skills need to be covered;
c) there are advantages in introducing some of the skills in a
specific module;
d) the exercises can be used to develop or revise chemistry;
e) the skills must be 'learned' rather than 'taught';
f) students need several opportunities to develop the skills;
g) appropriate exercises can be demanding, but are also fun for
both students and lecturers.
I would like to thank many colleagues from York, Heriot-Watt and Manchester Universities, who have helped me in developing and delivering a range of exercises in transferable skills, especially John Garratt and Sara Shinton, and the British Council for supporting my participation in 12ACC.
Bailey, P.D. 1997. 'Coaxing chemists to communicate', U. Chem. Ed., 1, 31-36.
Bailey, P.D. 1999. 'Assessment of Chemistry Degrees', U. Chem. Ed., 3, 64-67.
Bailey, P.D. 2001. 'Teaching chemists to communicate? Not my job!', University Chemistry Education, 5, 80-86.
Bailey, P.D. 2005. 'The poor Raymond investigation: a team work exercise to inspire new students', Proceedings of The Science Learning and Teaching Conference 2005, pp.53-58, The HE Academy.
Bailey, P.D. 2007. 'The poor Raymond investigation', Interchange (TLA News, University of Edinburgh), Spring 2007, pp. 12-15.
Bailey, P.D. & Shinton, S.E. 1999. Communicating Chemistry: A Teaching Manual for University Teachers: pp.1-141, Royal Society of Chemistry (tutors' guide to 10 exercises, available from RSC Education Department).
Dearing, R. (chairman) 1997. Higher Education in the learning society (report of the National Committee of Inquiry into Higher Education), Crown Copyright, 1997.
Duckett, S.B., Garratt, C.J. & and Lowe, N.D. 1999. 'Key skills: what do chemistry graduates think?', U. Chem. Ed., 3, 1-7.
Fry, H., Ketteridge, S. & Marshall, S. (eds.) 2003. A Handbook For Research & Learning in Higher Education: Enhancing Academic Practice (2nd edition): 'A communication course for chemists', pp. 268-270, Kogan Page, 2003.
Mason, G. 1998. Change and Diversity: The challenges facing chemistry higher education" Royal Society of Chemistry (in association with the Council for Industry and Higher Education), pp. 1-58, Royal Society of Chemistry, 1998.
Quality Assurance Agency 1998. General Guidelines for the Academic Review of Bachelors Honours Degree Courses in Chemistry, (published by) Quality Assurance Agency for Higher Education, 2000. URL: http://www.qaa.ac.uk/academicinfrastructure/benchmark/honours/chemistry.asp
Quality Assurance Agency 2007. Subject benchmark statements: Chemistry (draft for consultation). URL: http://www.qaa.ac.uk/academicinfrastructure/benchmark/statements/drafts/Chemistrydraft07.asp