Chemical Education Journal (CEJ),
Vol. 10, No. 2 /Registration No. 10-14/Received May 21, 2008.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
Microscale chemistry involves conducting experiments using significantly reduced quantities of chemicals and miniature labware. Students' exposure to potentially toxic chemicals and the probability of fire explosions are also greatly reduced. This article presents the development of microscale chemistry experimentation for the Malaysian Form Five Chemistry syllabus focusing on several experiments involving rates of reaction, ethanol and ester preparation, oxidation and reduction reactions, exothermic and endothermic reactions and soap preparation. Comparisons between the traditional (macroscale) and microscale experiments in terms of amount of chemicals used, amount of chemical waste produced and time savings are highlighted.
Microscale chemistry is a pollution preventive method and can be an effective tool to demonstrate chemistry concepts to students. It is carried out by using drastically reduced amounts of chemicals (as little as 25 to 100 milligrams of solids and between 100 to 200 microliters of liquids), safe and easy manipulative techniques and miniature labware (Singh et al., 1999). Precision or accuracy of experiments is not compromised. It is also recognized as small scale chemistry by IUPAC and can also be used as a tool to design new lab activities. The experiments are simple to conduct and the apparatus are affordable to be obtained by public schools and any educational institution. The benefits of this approach include cost and time savings, improved safety, pollution prevention and environment-friendly. It also brings theory closer to the laboratory and promotes hands-on learning experience, fosters creativity, improves skills in handling equipment and also changes students' views on the environment (Cooper et al., 1995, Bradley, 1999, Kelkar et al., 2001, Bradley, 2002, Tallmadge et al., 2004).
Most of the Form Four (16 years old) chemistry experiments
in the Malaysian secondary school chemistry syllabus can be adapted
to the microscale approach. The Form Four chemistry curriculum
focuses on basic chemistry concepts such as structure of the atom,
chemical formulae and equations, periodic table of elements, chemical
bonds, electrochemistry, acids and bases, salts and manufactured
substances (Curriculum Development Center,
2004). There are about 40 Form Four experiments that have
been developed using the microscale approach (Abdullah
et al., 2005). Most of the experiments have been designed
using the Microchemistry Kit (Centre for Research and Development
in Mathematics, Science and Technology Education (RADMASTE Centre),
South Africa) and other small volume glassware as suggested in
the Microchemistry Equipment Kit (National Microscale Chemistry
Center, USA). The combination of plastic wares and glassware are
used in designing the experiments since many experiments in the
Form Five curriculum require heating. In some cases, combined
use of traditional equipment and microscale apparatus was necessary.
The basic items include a comboplate (combination 24-well and
96-well plate), syringes, propettes, vials and small scale glassware
such as 10-mL beaker, 10-mL conical flask. Figure
1 shows the items in the Microchemistry Kit.
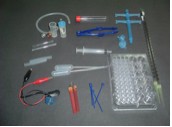
Microscale experiments can help teachers carry out experiments efficiently. Apart from reducing cost of chemicals used, chemical wastes and time spent, the objective of the experiments can be achieved successfully (Abdullah et al., 2005). Dangerous experiments such as the determination of the empirical formula of copper(II) oxide which involves reduction of the oxide by hydrogen gas has been carried out by teachers as a demonstration or has been discussed theoretically most of the time. Using this approach, this experiment can be done safely and individually by the students. Quantitative experiments which involve measurement of volume, weight or height can also improve students' skills in handling equipment and with greater care. In acid-base titrations, in order to get good data, the student needs to conduct titrations carefully using 2-mL microburettes. Kelkar & Dhavale (2000) reported that undergraduate students performed experiments with more care and their skills in handling the equipment were markedly improved after adoption of this technique in their laboratory.
Teachers and students have also given positive responses to this approach. Based on the workshops that have been conducted, teachers had a positive view towards the microscale experiments that have been developed (Abdullah et al., 2006a & Abdullah et al., 2006b). They perceived that the microscale approach is suitable, economical, student centered, interesting and time saving, stimulates enjoyment in learning chemistry and reduces wastes. The experiments are also portable. Abdullah et al. (2007) reported that most of the students agreed that by conducting microscale experiments individually, they could understand the concepts and experiments better, had fun and were interested to do more experiments. However, studies on high school students by McGuire et al. (1991) had shown that overall there was a preference for the macro version over the micro version for three experiments conducted.
Encouraged by these positive responses from teachers and students, the microscale approach was extended to the Form Five chemistry experiments. This article presents the development of microscale experimentation for several selected experiments in the Form Five (17 years old) chemistry curriculum. The comparison between traditional and microscale in terms of the apparatus used, quantity of chemicals used, waste produced and time spent will be highlighted.
Development of the Experimentation
The microscale chemistry experiments have been developed based on the Form Five Integrated Curriculum for secondary schools (KBSM) chemistry syllabus. There are about 35 experiments based on the topics of the KBSM chemistry syllabus (Curriculum Development Center, 2006). However, school trials for these experiments have not been conducted. The apparatus used and descriptions of the experiments developed are as follows:
(i) Effect of surface area on the rate of reaction
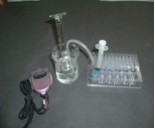
The objective of this experiment is to investigate the effect of the surface area of zinc metal on the rate of its reaction with sulphuric acid. The experiment is carried out using granulated and powdered zinc. The volume of gas produced from the reaction is measured at definite time intervals. The traditional set-up consists of a 50-mL burette, basin, retort stand, delivery tube, stop watch and a 250 mL conical flask. In the microscale set-up, a 10 mL measuring cylinder, a 100 mL beaker, U-tube, comboplate, lid 1, a stop watch and a syringe are used. For the microscale set-up, the 10 mL measuring cylinder is used instead of a 50 mL burette to collect the gas. The measuring cylinder is filled with water and turned upside down in the beaker in order to measure the volume of the gas produced. The well in the comboplate replaces the conical flask as a reaction vessel. The syringe is used to add the acid to zinc. The gas flows through a U-tube to the inverted measuring cylinder as shown in the microscale set up in Figure 2. The rate of reaction is measured according to the rate of gas produced.
The traditional set-up used about 2 g of granulated/powdered
zinc and 40 mL of sulphuric acid whereas the microscale set-up
used only 0.05 g of granulated/powdered zinc and 0.5 mL of 1 mol
dm-3 sulphuric acid. The traditional experiment is usually conducted
by a group of students whereas the microscale experiment can be
done safely on an individual basis.
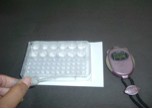
(ii) Effect of concentration on the rate of reaction
The reaction involved in this experiment is between sodium
thiosulphate and sulphuric acid. The aim of this experiment is
to investigate the effect of the sodium thiosulphate concentration
on the rate of the reaction. The rate is estimated from the reciprocal
of the time taken for a fixed mass of sulphur to be formed. With
a longer time taken, the rate of reaction is lower. Different
concentrations of sodium thiosulphate solutions but of similar
volumes are mixed with acid solutions having the same volume and
concentration. In this experiment, the comboplate and thin stemmed
propettes are used in the microscale set-up (Figure
3). The traditional set-up used a 150 mL of conical flask
and 50 mL measuring cylinder. For the microscale set-up, the smaller
wells in the comboplate are used instead of the conical flask
as a container to mix the sodium thiosulphate solution and sulphuric
acid. For the traditional set up, the total volume of the reacting
mixture is about 50 mL. In this case, the microscale approach
promotes significant reduction of chemicals used and waste produced.
(iii) Preparation of ethanol and esters
The microscale distillation set consists of similar apparatus as the traditional distillation set but with smaller volumes and sizes such as 100 mL round bottom flask, 250 mL beaker, small fractionating column, small Liebig condenser, small receiver, 10 mL conical flask and retort stand.
Fermentation of glucose involves the reaction between glucose and yeast which is carried out in a conical flask. Subsequently the distillation of the filtrate from the fermentation process can be carried out on a smaller scale for the microscale set up. The traditional distillation process requires about 60 - 80 mL filtrate, but this can be reduced to 5 - 10 mL for the microscale technique. The microscale technique will produce about 0.25 to 1 mL of distillate. It is important to maintain the temperature at 78 - 80 oC in order to get pure ethanol.
The apparatus used for ester preparation is quite similar with the distillation set up for ethanol except for the use of a tap funnel and an oil bath. The volume of absolute ethanol, glacial ethanoic acid and concentrated sulphuric acid used are about 10% of the suggested volume in the traditional scale and the volume of ester collected is about 3 to 4 mL. Both experiments can be conducted one set per group by microscale technique compared to only one set per class by traditional technique.
(iv) Transfer of electrons at a distance
The aim of the experiment is to investigate a redox reaction
between iron(II) sulphate and acidified potassium manganate(VII)
where the reactants are separated. Traditionally, this experiment
is conducted using a glass U-tube, a retort stand, graphite electrodes,
connecting wire with crocodile clips and galvanometer/multimeter
(Figure 4a). In the microscale set
up, a silicone U-tube is used with 2 microstands, pencil leads,
connecting wire with crocodile clips and galvanometer/multimeter
(Figure 4b).
(a) (b)
Sulphuric acid was used as a salt bridge to allow the transfer of ions between the reactants, an oxidising agent and a reducing agent, which are placed in separate arms of the U-tube. Several solutions which have been suggested as oxidising agents include potassium manganate(VII), potassium dichromate and bromine water whereas the reducing agents suggested include potassium iodide and iron(II) sulphate.
A major problem faced when conducting this experiment traditionally was the length of time needed for the reaction to complete. To ensure that oxidation and reduction occur in both solutions used, students have to observe colour changes such as the disappearance of the deep violet colour of manganate(VII) ions. Completion of the experiment takes about 15 minutes with the microscale set-up compared to about 60 - 120 minutes traditionally since less reactants are used. Thus it promotes time savings and reduction of chemicals consumed by using smaller amounts of solutions and more dilute solutions. The students are also able to conduct the experiment individually.
(v) Heat of displacement reaction
The heat of displacement is determined by adding a more electropositive metal such as zinc in excess to a copper(II) sulphate solution. This experiment can be done quickly for both traditional and microscale set ups. The microscale set-up used the wells of the comboplate instead of polystyrene cup as a container to mix the reagents.
(vi) Soap preparation
For the preparation of soap, a 10 mL measuring cylinder, a 10 mL beaker, microspatula, tongs, a 40 mm filter funnel, 55 mm filter paper, a microburner, a microtripod stand and a 25 mL conical flask are used in the microscale set up. The traditional set-up consists of similar apparatus but in larger sizes. The process of soap preparation involves heating a mixture of vegetable oil with concentrated sodium hydroxide (saponification), addition of sodium chloride (precipitation of soap), rinsing with water (removal of glycerol and excess sodium hydroxide), filtration and drying the soap.
In the microscale experiment, the volume of palm oil and sodium hydroxide used is about 10% of the suggested volume in the traditional set up. The weight of soap formed is about 0.1 to 0.2 g which is sufficient to conduct a physical test for soap. In this experiment, the reduction of chemicals used in the microscale technique provides significant time savings for the heating, filtration and drying steps.
Comparisons between traditional (macroscale) and microscale experiments
Tables 1, 2 and 3 give a comparison for the amount of chemicals used, amount of waste produced and time used between the microscale technique and that of the traditional technique for selected Form Five experiments that have been discussed. It has been shown that with the microscale approach, wastes are reduced up to 87 %, quantities of chemicals consumed are reduced up to 81% and time spent is saved up to 50%. Comparison between traditional and microscale experiments for several experiments in the Form Four chemistry syllabus has also shown that microscale chemistry reduces wastes up to 88%, chemical costs up to 87% and saves up to 66% of time spent.
Conclusions
Several selected Form Five experiments in the Malaysian secondary
school chemistry syllabus have been developed based on the microscale
chemistry approach. With this approach, there has been a reduction
in chemicals consumed and chemical waste produced as well as in
time spent conducting the experiments. Experiments can also be
conducted individually by students.
Abdullah, M., Mohamed, N and Ismail, Z. (2007). The effect of microscale chemistry experimentation on students' attitude and motivation towards chemistry practical work. Journal of Science and Mathematics Education in Southeast Asia, 30 (2), 44-72.
Abdullah, M., Mohamed N. and Ismail Z., (2006a). Introducing microscale experimentation to chemistry preservice teachers. In: O. Osman, and Z. A. Sanusi (Eds), The ASAIHL Conference on Education for Sustainable Development: Proceedings of Conference, 178-188.
Abdullah, M., Mohamed N. and Ismail Z., (2006b). Secondary school teachers feedback on microscale chemistry experimentation. In: Y. J. Lee, A. L. Tan, and B. T. Ho (Eds), Proceedings of the International Science Education Conference ISEC Singapore, 55-65.
Abdullah, M., Ismail Z. and Mohamed N., (2005). Microscale Experimentation in Teaching Chemistry. In: M. Ismail, S.Osman, and H.M.Yunus (Eds), Proceedings for Seminar Pendidikan JPPG 2005 - Education for sustainable development, Penang: Universiti Sains Malaysia, 96-103.
Bradley, J. (1999). Hands-on practical chemistry for all. Pure Applied Chemistry, 71 (5), 817-823.
Bradley, J.D. (2002). Small-scale chemistry. Chemistry International, 24 (3). http://www.iupac.org/publications/ci/2002/2403/smallscalechemistry.html accessed 1 February 2008.
Curriculum Development Center (2006). Chemistry Form Four Curriculum Specifications: Integrated Curriculum for Secondary Schools, Ministry of Education, Malaysia.
Curriculum Development Center (2004). Chemistry Form Four Curriculum Specifications: Integrated Curriculum for Secondary Schools, Ministry of Education, Malaysia.
Cooper, S., Conway K. and Guseman, P., (1995). Making The Most of Microscale: Using microchemistry as a tool to transform teaching. The Science Teacher, 65 (1), 46-49.
Kelkar, S.L., Dhavale D.D. & Chandwadkar J.G. (2001). Microscale experiments in Chemistry: The need of the new millenium. Resonance, 15-21.
Kelkar, S.L. and Dhavale D.D., (2000). Microscale experiments in Chemistry: The need of the new millenium, Resonance, 24-31.
McGuire, P., Ealy J. & Pickering M., (1991). Microscale laboratory at the high school level: Time efficiency and student response. Journal of Chemical Education, 68 (10), 869-871.
Singh, M.M., Szafran Z. & Pike R.M., (1999). Microscale Chemistry and Green Chemistry: Complementary Pedagogies. Journal of Chemical Education, 76 (12), 1684-1686.
Tallmadge, W., Homan M., Ruth C. and Bilek G., (2004). A local pollution prevention group collaborates with a high school intermediate unit bringing the benefits of microscale chemistry to high school chemistry labs in the Lake Erie watershed, Chemical Health & Safety, 11, 30-33.
Table 1: Comparison in terms of amount of chemicals used for selected
Form Five experiments (per 30 students)
| Experiments | Chemicals | Traditional Quantity (g or mL) |
Micro Quantity (g or mL) |
| Effect of surface area on the rate of reaction | sulphuric acid | 400 mL | 30 mL |
| granulated/powdered zinc | 80 g | 3 g | |
| Effect of concentration on the rate of reaction | sodium thiosulphate | 1160 mL | 15 mL (1080 drops) |
| sulphuric acid | 200 mL | 15 mL (1200 drops) |
|
| Ester preparation | absolute ethanol | 600 mL | 225 mL |
| glacial ethanoic acid | 400 mL | 150 mL | |
| concentrated sulphuric acid | 200 mL | 75 mL | |
| Transfer of electrons at a distance | sulphuric acid | 160 mL | 15 mL |
| acidified potassium manganate (VII) | 40 mL | 7.5 mL | |
| potassium iodide | 40 mL | 7.5 mL | |
| Heat of displacement | Copper (II) sulphate | 200 mL | 30 mL |
| Zinc powder | 24 g | 2.4 g | |
| Heat of neutralization | hydrochloric acid | 200 mL | 30 mL |
| sodium hydroxide | 200 mL | 30 mL | |
| Soap preparation process | palm oil | 80 mL | 30 mL |
| sodium hydroxide | 400 mL | 150 mL | |
| sodium chloride | 20.8 g (8 spatulaful) |
9.9 g (150 microspatula) |
|
| Total | 4280 mL | 810 mL |
Table 2: Comparison in terms of waste produced for selected
Form Five experiments (per 30 students)
| Experiments | Waste produced | |
| Traditional Quantity (g or mL) |
Micro Quantity (g or mL) |
|
| Effect of surface area on the rate of reaction | 400 mL (Zn + H2SO4) |
30 mL (Zn + H2SO4) |
| Effect of concentration on the rate of reaction | 2000 mL (sulphur precipitate) |
45 - 60 mL(sulphur precipitate) |
| Ester preparation | 400 mL (unreacted C2H5OH, CH3COOH & conc H2SO4) |
60 mL (unreacted C2H5OH, CH3COOH & conc H2SO4) |
| Transfer of electrons at a distance | 240 - 320 mL (KI + KMnO4 + H2SO4) |
30 - 45 mL (KI + KMnO4 + H2SO4) |
| Heat of displacement | 160 mL CuCl2 24 - 32 g unreacted Zn 30 mL |
CuCl2 15 g unreacted Zn |
| Heat of neutralization | 400 mL ( NaCl + H2O) |
60 mL ( NaCl + H2O) |
| Soap preparation process | 560 - 640 mL (glycerol + excess NaOH) |
270 - 300 mL (glycerol + excess NaOH) |
| Total | 4160 mL | 525 mL |
Table 3: Comparison in terms of * time spent
conducting selected Form Five experiments
| Experiments | Time (minutes) | |||
| Traditional | s | Micro | s | |
| Effect of the amount of catalyst on the rate of reaction | 56.7 min | 1.5 | 25.3 min | 2.5 |
| Effect of concentration on the rate of reaction | 25.3 min | 2.5 | 20.3 min | 2.5 |
| Ester preparation | 40.3 min | 2.5 | 25.3 min | 2.5 |
| Transfer of electrons at a distance | 80.0 min | 5.0 | 17.7 min | 2.5 |
| Heat of displacement | 6.3 min | 1.5 | 5.3 min | 1.5 |
| Heats of neutralization of acids and alkalis of different strength | 22.0 min | 2.0 | 21.0 min | 1.0 |
| Soap preparation process | 82.0 min | 2.0 | 29.7 min | 1.5 |
| Total | 312.6 min | 144.6 min | ||