Chemical Education Journal (CEJ),
Vol. 10, No. 2 /Registration No. 10-17/Received May 21, 2008.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Introduction
According to the investigation made by the Ministry of Education,
Culture, Sports, Science and Technology of Japan (MEXT) in 2004,
chemistry is the most unpopular subject among high school students.
Students seemed to find chemistry uninteresting, difficult to
understand, and they think it is useless to study chemistry.
There are two main causes for the bad reputation of chemistry. One is the deteriorated image of chemistry. Chemicals are viewed as the main cause of many environmental problems by the general public. To improve the bad image of chemistry, it is suggested to introduce the concept of green chemistry (GC). The other cause of chemistry for being least popular is the way by which chemistry is taught in some high schools where chemistry is taught without experiments, as experiments are often thought to be less effective for the preparation of the entrance examination to universities. To make chemistry interesting, attractive experiments are essential in chemistry classrooms.
Microscale Laboratory
Microscale laboratory is environmentally benign as the amount
of reagents, waste, energy and cost can be reduced dramatically,
and exposure to potentially toxic chemicals is diminished. Some
aspects of Green chemistry can be taught through microscale laboratory.
We have developed various microscale experiments on the following
basic topics: 1) Acids and bases, 2) Properties of metal ions,
3) Chemical equilibriums, 4) Galvanic cells, 5) Electrolysis,
6) Ion exchangers, 7) Reaction rate, 8) Titrations, 9) Properties
of amino acids, 10) Ion exchangers, etc. Low price glass and plastic
wares such as 12- and 24-well plates, vials, syringes and droppers
are used. These are available in any country at low prices.
A questionnaire on microscale experiments was administered to both students and teachers.1) The results revealed the following characteristics of our microscale experiments:
1. Many reactions are accompanied by changes in color or evolution of gases. They can be observed very clearly with these cell plates. As all the original reactants are placed in the corresponding wells, the color of product can be compared easily with those of reactants.
2. The procedures are simple and require little explanation. Therefore, the time for explanation before the experiments is shortened considerably.
3. All experiments can be done in a short time.
4. Students can carry out experiments without failure. For example, the plates would not fall down, whereas beakers and test tubes may fall down, especially when electrodes are connected. Students would not make any mistake in the selection of solutions in the wells of the plates, whereas mistakes may happen when beakers or test tubes are used and various solutions are handled.
5. The experiments are reproducible.
Low priced glass and plastic wares such as 12- and 24-well plates, vials, syringes, droppers are used. These are available in any country at low prices.
An Example of Microscale Experiments: Electrolysis
1. Measurement of the volumes of gases evolved by the electrolysis
An example of a microscale experiment is the electrolysis using
a microscale Hoffman apparatus shown in Figure
1.2) It is constructed
with 1 mL syringes without plungers, disposable stopcocks and
a 12-well microplate. Pins are pierced into the syringes at the
graduation mark of 0.80 mL. They serve as electrodes.
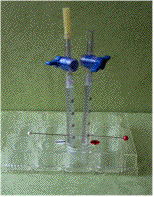
Figure 1: Microscale Hoffman Apparatus
for Electrolysis consisting of 1 mL syringes, disposable stopcocks,
pins and a 12-well plate.
3 mL of saturated sodium carbonate solution is added in the well.
Connect a 2 mL syringe to one disposable stopcock via rubber tubing,
and the plunger of the syringe is pulled so that the 1 mL syringe
is filled with sodium carbonate solution. The stopcock is then
closed and the 2 mL syringe is removed. The other 1 mL-syringe
is also filled with sodium carbonate solution in a similar way.
When the electrodes are connected to a 9 V dry cell and electrolysis
is started, the formation of bubbles at the electrodes is observed.
In a few minutes, it is shown clearly that the volume of gas formed
at cathode is twice as much as that formed at the anode. Finally,
the electrolysis stops automatically, when the volume of the gas
at the cathode is 0.80 mL and that at the anode is 0.40 mL. This
result corresponds to the electrolysis of water by the following
equation:
2 H2O ![]() O2 + 2 H2
O2 + 2 H2
An ordinary Hoffman apparatus costs more than $200, and is difficult to handle. More than 500 mL of solution is often necessary. However our microscale apparatus can be constructed at less than $10 and can be handled very easily. Only 3 mL of solution is needed. We can let every student carry out electrolysis with the microscale Hoffman apparatus.
2. Examination of the reactions occurring at electrodes
by the electrolysis of various aqueous solutions 3,4)
Electrode reactions at the anode and cathode can be examined through
microscale electrolysis experiments. The electrolysis is carried
out in wells of a 12-well plate placed on a work sheet shown in
Figure 2. 2 mL of sodium sulfate solution
and 5 drops of bromothymol Blue (BTB) pH indicator solution are
added into the corresponding well. The well is separated into
two parts (the anode and the cathode compartments) with a piece
of filter paper.
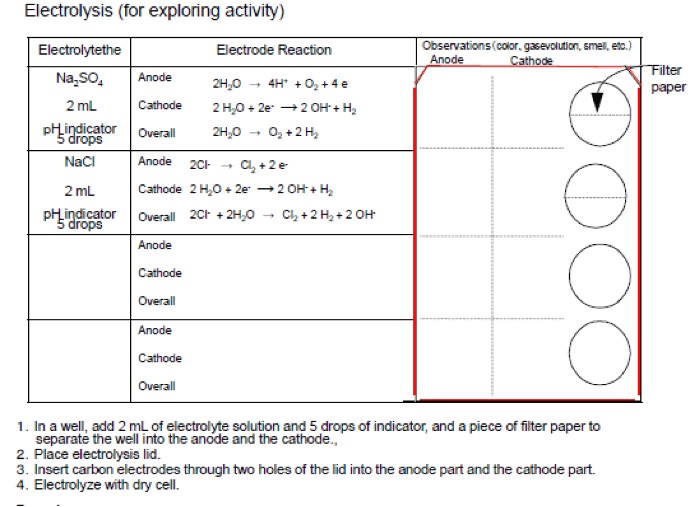
Figure 2 Students worksheet for electrolysis of various electrolytes.
A12-well plate is placed on the right side of the sheet shown
by red color. In the lower two lines, students can explore electrolysis
of solutions of electrolytes they choose.
Carbon rods are used as both anode and cathode. They are placed
in each side of the filter paper in the well. When the electrolysis
is started by connection to a 9 V dry cell, evolution of gas is
observed at the surface of electrodes, and clear color changes
are observed immediately as shown in Figure
3. Before electrolysis, the color of the solution is yellowish
green as it is neutral. After electrolysis, the solution at the
anode becomes yellow as hydrogen ions are produced, and that at
the cathode becomes blue due to the formation of hydroxide ions.
It coincides with the chemical equations shown on the worksheet
in Figure 2. Students can record the observations on the sheet.


Figure 3 Color changes during the electrolysis of sodium sulfate solution containing BTB. A) Before electrolysis, solution in both anode and cathode compartments is yellowish green. B) After electrolysis, the solution in anode compartment is yellow, while the color of the solution in cathode is blue.
During the electrolysis of sodium chloride solutions containing red food coloring, clear color change is observed as shown in Figure 4. Fading of the red color takes place due to the oxidation by chlorine formed at the anode.


Figure 4 Color changes during the electrolysis of sodium chloride solution containing red food coloring. A) Before electrolysis, solution in both anode and cathode compartments is red. B) After electrolysis, the solution in anode compartment is colorless, while the color of the solution in cathode remains red.
In a few seconds, the smell of chlorine can be noticed. These observations are in accordance with the chemical equations shown in the work sheet.
Extract of red cabbage can be used as pH indicator and as dye solution.
The lower two lines of the worksheet in Figure 2 are left vacant. Students can try the electrolysis of other electrolytes such as copper sulfate, copper chloride, magnesium sulfate, etc. They can add some pH indicators and food coloring to these electrolyte solutions. They are advised to write equations of the electrode reactions and to presume the phenomena which would be observed by the electrolysis. By comparing the experimental results with the presumption, students can deepen their understanding.
Discussion
Students are very happy to carry out these microscale experiments,
because they are interesting. Students appreciate that microscale
chemistry is environmentally benign. As microscale experiments
can be carried out in a short time, students can perform some
other challenging and exploring activities instead of the cookbook
experiments. Exploring activities can increase students' positive
attitude and nurture creative thinking.
Now many Japanese teachers are interested in introducing microscale laboratory. They appreciate that microscale laboratory is environmentally benign. Microscale laboratory helps students' understanding of GSC and is effective in creating a positive image of chemistry.
Acknowledgements
This work is supported by Grants-in-Aid for Scientific Research
(No. 17011005 and No. 19500716) from the Ministry of Education,
Culture, Sports, Science and Technology of Japan.
References
1. Quantitative analyses of the answers
of 900 students and 14 chemistry teachers of 11 high schools are
described in Ogino, K., Shoji, K., Kon, K., Tajima, T., Fujikawa,
T., Tkahashi, M., Chemistry and Education (Japan), 49,
169-171, 2001.
2. Ogino, K., Chemistry and Education (Japan), 55, 82-83, 2007.
3. Ogino, K. and Shoji, K., Chemistry and Education (Japan), 46, 742-743, 1998.
4. Ogino, K., Chemistry and Education (Japan), 55, 336-339, 2007.