Chemical Education Journal (CEJ),
Vol. 10, No. 2 /Registration No. 10-11/Received November 21, 2007
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
Web-based materials were prepared using airborne particle data
for teaching materials to connect the world in vitro to natural
phenomena and global environment. Airborne particles have been
collected since 2000 in Gifu situated in the middle of the main
Island of Japan. The particles were observed by a scanning electron
microscope (SEM), analyzed by an energy dispersive X-ray spectrometer
(EDX), and classified into soil, pollen, sea-salt, soot and some
cosmic dust. It is suggested that the annual movement of soil
particles containing calcium is strongly related to Kosa (yellow
sand) events occurred from sand storms at desert areas in China.
The particles were collected 50 km distant from the sea. Sea salt
crystals were observed still in this place, especially after typhoon
passed through this area. SEM images and EDX spectra of these
particles are presented on the website as well as the movements
described above. These website materials aim to help teachers
in primary and junior high schools to teach pupils and students
about plants and pollination, solution and crystals, weather,
the earth and living things, and the environment in science classes.
The materials also aim to make pupils and students aware of the
circulation of substances in the atmosphere.
Keywords: website materials, teaching materials, airborne
particles,environmental education, chemical education, science
education
Since the 1997 Kyoto Protocol, the threat of global warming has been increasing, because reducing greenhouse gas emission has not proceeded satisfactorily. In Siberia, permafrost has started to melt because of increasing temperature; methane gas, which is sealed in it and which has more than twenty times larger greenhouse effect than carbon dioxide, has started to be released. The global warming seems to accelerate dramatically and soon.
Descriptions about global environment have appeared in science textbooks for elementary school and junior high school after 1992 Rio Declaration on Environment and Development. However, the environment crisis has not been treated so seriously until recent years. In the circumstances, science education has to play an important role in teaching global environment to younger generations. We have to be sure to make pupils, students, and teachers to be aware of the reality.
The learning contents of one of the latest textbooks in science education in elementary school and junior high school in Japan are shown briefly in Tables 1 and 2. In Japan, science education starts from the third grade in six years of elementary school. At the third grade the contents are constructed to turn the children's interests from their personal world to the objective world.
Table 1. Contents of science
education in elementary school in Japan
|
|
contents |
|
|
plants and small insects surrounding us |
|
|
seasonal changes of plants, stars, and life of living things, transformation of water, temperature and volume |
|
|
budding and growing of plants, pollen, birth of life, weather, flowing water, natural disaster, solutions and crystallization |
|
|
burning (oxygen, carbon dioxide),
mechanism of our bodies, properties of solution (acid, base,
chemical reactions), the earth, volcanism, our environment |
Table 2. Contents of science education in junior high school
in Japan
|
|
contents |
|
|
light, sound, force, electricity, properties of various substances around us, chemical reactions and applications, science techniques and human being |
|
|
plants, animals, and their lives, the globe, the atmosphere, weather, nature and human being |
Therefore, learning materials are plants, insects, and some other
things found outdoors and close to children. Science education
of junior high school is divided into two fields, one of which
includes physics and chemistry and the other biology and earth
science. Students learn both fields in three years. In higher
grades and in junior high school children learn objectivity and
deepen their scientific knowledge; at the same time they realize
the importance of the global environment and the relation between
nature and human beings. In the objective world, for the field
of chemistry, we mainly deal with substances and phenomena in
vitro. We would like to use substances and phenomena which are
familiar in our daily life. These are, however, sometimes too
complicated to understand, so that very simple substances and
phenomena in vitro are selected for teaching. Thus, students seem
to have an impression that the world in vitro is not related to
natural outside world. We wished to make some learning materials,
which connect to natural phenomena and the global environment.
Here, we present web-based materials made for this purpose. The
materials are developed from our airborne particle observation.
We believe it is very useful for science teachers, especially
in junior high school, to deal with the environment and the circulation
of substances in the atmosphere relating to all the fields.
Airborne particles have been collected on an adhesive tape
attached on a glass plate set vertically on the rooftop of our
faculty building (26 m high) every week in Gifu (35.46oN,
136.74oE), Japan, being about 50 km away from the sea.
These particles were observed by an scanning electron microscope,
SEM (Hitachi S-4300), equipped with energy dispersive X-ray spectrometer,
EDX (Horiba EMAX) and were classified into soil, pollen, sea-salt,
and soot.
SEM images and EDX spectra were saved independently and these images were united with Addpic [1]. Since these images were saved as bitmap data, the files were too large to handle on the webpage. Therefore, all the images were transformed to jpg files and processed into HTML by one of free softwares [2]. The other files were processed into HTML files by using TeraPad [3] and Homepage manager [4].
The first page shows six windows to get into the pages of sea-salt, pollen, soil, soot, spherical particles, and characteristic-shaped particles.
For the sea-salt page, we showed several SEM images
of particles having characteristically cubic or sometimes rectangular
parallelepiped crystalline shape and various kinds of shapes,
as given in Figs. 1 and 2.
These sea salt crystals were found especially after a typhoon
passed this area. We showed the relation between the number of
sea-salt particles found each week and the everyday changes of
atmospheric pressure and rainfall, as shown in Fig.
3.
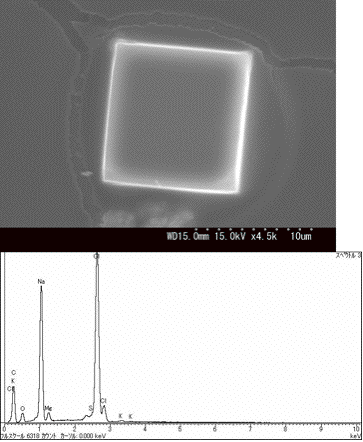
Fig. 1. A cubic sea-salt crystal and its EDX spectrum.
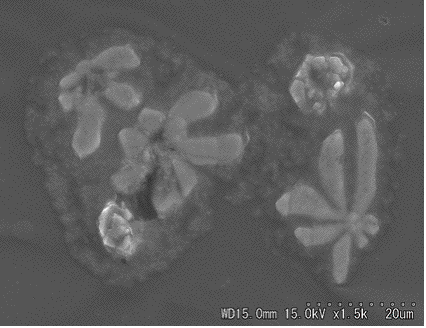
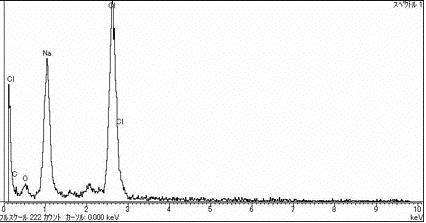
Fig. 2. Different shape of sea-salt particle and its EDX spectrum.
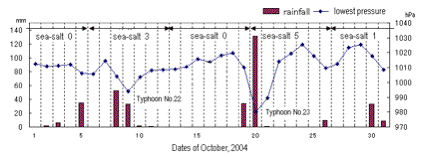
Fig. 3. One of figures about the number of sea-salt particles
found each week and the everyday changes of the lowest atmospheric
pressure and rainfall. The real figures in web pages are written
in Japanese.
About thirty kinds of pollen and their EDX spectra were presented
among ninety-six pollen grains we found in three years from 2003
to 2005. Japan cedar pollen is also shown in the pages, as given
in Fig. 4, which is most notorious
in Japan because of its pollinosis.

Fig. 4. Japan cedar pollen grains and its EDX spectrum.
The distribution of these grains depending on months was presented as shown in Fig. 5. Some of the unidentified grains were also included.
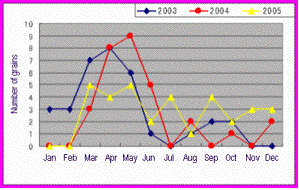
Fig. 5. The distribution of ninety-six pollen grains we collected
from 2003 to 2005. The real figure in the web pages is written
in Japanese.
Soil particles may be classified into rock-forming minerals.
We presented twenty soil particles including several particles
identified as quart and feldspar. However, since the suspended
particles are often a mixture of those minerals, we did not make
much effort for their identifications. We obtained the proportions
of the number of particles containing some elements to the total
number of the soil particles. Soil particles are mainly silicates,
but sometimes we found particles consisting of CaCO3
and CaSO4 crystals, as shown in Fig.
6. Therefore the proportion of the particles containing Si
is not necessarily 100 %.

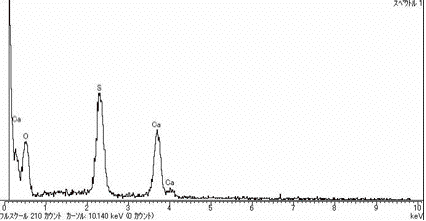
Fig. 6. A particle containing CaSO4 crystals
and its EDX spectrum.
It is known that calcium content of desert soil in China is higher than that in our land soil [5-7]. We reported in 2007 that the 2002 movement of the proportion of soil particles containing Ca agreed well with the change of the number of days when yellow sand (Kosa) was detected in Japan [8,9]. It also has been reported that yellow sand neutralizes sulfuric acid caused by using fossil fuel in atmosphere [10]. The movement described above also agreed well with the change of the proportion of soil particles containing sulfur [8]. These suggest that the prevailing westerlies bring the soil particles of Kosa from China to Japan; on the way to Gifu, a part of CaCO3 in Kosa changes to CaSO4 in a reaction with H2SO4. Thus, the proportion of soil particles containing S shows similar movement as the proportion of soil particles containing Ca. We referred these movements to show an actual chemical reaction and the circulation of substances in atmosphere.
In the part of soot, we showed fourteen soot particles on the
pages. Some of them are porous as shown in Fig.
7, which indicates that this is a trace of decomposition of
hydrocarbon and oxidation of sulfur containing in heavy oil used
for combustion furnace. Several soot particles were collected
for comparison from diesel vehicles by exposing the adhesive tape
to their exhaust gas. These have cloud-like shapes where many
small particles were gathered. One of them is also shown in the
pages.

Fig. 7. A porous soot particle and its EDX spectrum
Eight spherical particles including pollen, soot, and soil
are shown on the pages. Among the thirty spherical soil and soot
particles we had collected between 2003 and 2005, only three soil
particles were classified as cosmic dust from their surface patterns
and compositions according to the definition [11].
These are presented on the pages. One of them is shown in Fig. 8.
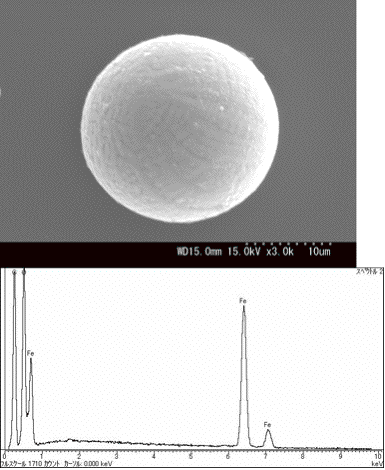
Fig. 8. Surface pattern of one of cosmic dust particles and its
EDX spectrum.
The present web-based materials [12] were prepared to provide teaching materials for science teachers in primary school and junior high school.
In the concrete, we suppose several sample fragments to use these teaching materials for the development of classes as below.
The EDX spectra show that all the particles consist of chemical elements. The students will recognize that not only chemical products but also all the matters consist of chemical elements lined up in a periodic table. This recognition is a significant step for students to understand substances chemically, which students start to tackle in junior high school. Next, the reaction of CaCO3 in soil particles with H2SO4 in acid mist makes students aware of chemical surroundings.
The SEM images and the EDX spectra of sea-salt crystals are also helpful for pupils and students in studying solution and crystallization as well as the constituents of matter. These particles are related with a low pressure generated on the sea. Furthermore, the fact may induce a question why typhoon occurs often in summer and autumn, about which pupils study in the parts of weather, water flow, and natural disaster in the fifth grade. These atmospheric phenomena should be a trigger to start to think of global environment.
The SEM images of a variety of pollen grains help pupils and students to understand botanical surroundings. These can be used by teachers to teach higher grade pupils about pollination and generation in contrast to a role of insects: What characteristics do the plants have, whose pollen grains were often collected in the air? Are those flowering plants? The variety of shapes of pollen grains can be helpful for the students in junior high school to notice the strategy for preservation of species in respect of plant morphology. On the other hand, the distribution of the pollen grains shown in Fig. 5 is useful for lower grade pupils to understand the relation between the seasons and plants.
For Kosa events, we can give some questions why low pressures occur often on desert areas in China in spring and why soil particles are lifted up. Similarly as the generation of typhoon, these questions are related deeply with phenomenological process how matters get warmed by heat or sun-light, which pupils start to learn and recognize in the forth grade. This question is extended to other questions why we have seasons and why it is hottest in August in Japan, while summer solstice is in June, which students study in junior high school.
Therefore, these uses make possible for teachers to teach pupils introductory science with thinking of the evolution to the phenomena on the earth. At the same time, these help science teachers in junior high school to teach students on the basis of understanding of the connection from science in a lab to global environment. We expect that students extend their thinking to the environment and furthermore to the global environment.
In order for science teachers to use these SEM images as teaching materials in their classes, many more interesting SEM images will be needed. There are not still enough SEM images of the particles shown in our web pages. Therefore, we are going to increase the number of SEM images. In future, we would like to make a database for airborne particles.
The presentation of the movements is still crude. It is necessary for us to keep updating the movements and developing the way of presentation from now on.
Concerning the global environment, there is a need for access
to other web materials and information of other researchers. Therefore,
we are planning to make a page showing the links.
References
[1] http://download.seesaa.jp/contents/win/graphic/soundt/04447/
[2] http://homepage3.nifty.com/metis/
[3]
http://www5f.biglobe.ne.jp/~t-susumu/
[4] http://hp.vector.co.jp/authors/VA014491/
[5] Y. Hseung and M. L. Jackson,
Soil Sci. Soc. Amer. Proc.,16,
294-297 (1952).
[6] S. Tanaka, M. Tajima, S. Sato, and Y. Hashimoto, Advances
in
X-ray Chemical Analysis Japan, 17, 253-264 (1986).
(in Japanese)
[7] M. Nishikawa, S. Kanamori, N. Kanamori, T. Mizoguchi, Sci.
Total
Environ., 107, 13-27 (1991).
[8] S. Sato, S. Tsuchikawa, Y. Higashi,
and A. Hiratsuka, Sci. Rep. Fac.
Educ. Gifu Univ. (Nat. Sci.) 31 (2007) 11-18.
[9] Japan meteorological Agency,
http://www.data.kishou.go.jp/obs-env/kosahp/kosa_data_index.html
[10] X. Dong, K. Sakamoto, W. Wang,
H. Liu, C. Thang, S. Niu, and Y.
Chen, J. Aerosol Res., Jpn., 14, 248-256 (1999).
(in Japanese)
[11] http://www.geocities.co.jp/CollegeLife-Labo/1501/work1.html
http://www.geocities.co.jp/CollegeLife-Labo/1501/mmi1.html
[12] http://www1.gifu-u.ac.jp/~edkagaku/sato/index2.html
CEJ Vol. 10, No. 2, Contents