22 Hachashmal St., P.O. Box 8349
Haifa 33145,
Israel
muha4
Chemical Education Journal (CEJ),
Vol. 11, No. 2 /Registration No. 11-9 /Received February 25, 2008.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
In this lab demonstration, an integrative chemical phenomenon
is demonstrated by means of electrolysis of salty water solutions.
In these experiments, the students can observe directly the cathodic
and anodic phenomena by using an acid-base indicator in the electrolysis
system, the ions produced at each electrode are identified, and
collect in a small balloon the gases that are produced during
the experiments, the gases produced are identified. In this article
we built a different simplified version of the conventional Hoffman
apparatuses for water electrolysis using disposable materials.
Electrolysis is a chemical change, specifically the decomposition of an electroactive substance induced by an electric current. Starting from the last century, the electrolysis of a solution has been widely demonstrated to the students in order to illustrate oxidation-reduction reactions as well as to demonstrate the usage of an external source of energy for driving non-spontaneous chemical reactions. On the other hand, it is sometimes used to catalyze reactions which are very slow at ambient conditions (1-3).
In our experiments, water is electrolyzed by using Na2SO4 as electrolyte support and a 9V battery or a 12V AC power supply. Electrode phenomena can be observed when an appropriated indicator is present in the electrolyzed solution. In chemistry, an indicator is a good mean to indicate the presence of a specific reagent or the occurrence of a chemical reaction. For example, in the current experiments a natural acid-base indicator is used to show the production of the hydroxide anion in the surrounds of the anode. By the other hand, as a result of the electrolysis of water, bubbles of gaseous hydrogen and oxygen are produced in the cathode and anode electrodes, respectively.
Hofmann apparatus is an apparatus for electrolyzing water, invented by August Wilhelm von Hofmann (1818-1892). It consists of three joined upright cylinders, usually glass. The inner cylinder is open at the top to allow addition of water and an ionic compound to improve conductivity, such as a small amount of sodium sulfate. A platinum electrode is placed inside the bottom of each of the two side cylinders, connected to the positive and negative terminals of a source of electricity. When current is run through Hofmann's apparatus, gaseous oxygen forms at the anode and gaseous hydrogen at the cathode. Each gas displaces water and collects at the top of the two outer tubes (1-3). In this article, a simple and feasible idea to introduce microscale electrolysis experiments by using accessible materials in the classroom is presented.
The construction of an inexpensive
T-Hoffman electrolysis apparatus
In 1982 Lloyd J. Hendricks and John T. Williams published their article in the journal of chemical education (2). In that article they presented a new apparatus to illustrate the electrolysis of water, the article describes the construction of an electrolysis cell (Fig. 1a). In our lab we built different size apparatuses to carried out the electrolysis of water (Fig. 1b,c), whose purpose was to reduce the volume of the solution and the quantity of the reagents used in the experiment collect the two gases (H2 and O2) which produced during the electrolysis (Fig. 1b, c). As you can see in the apparatuses presented in the figure 1, the volume of the solution could be reduced from 500 mL (Fig. 1 a, b) to 20 mL (Fig. 1d).
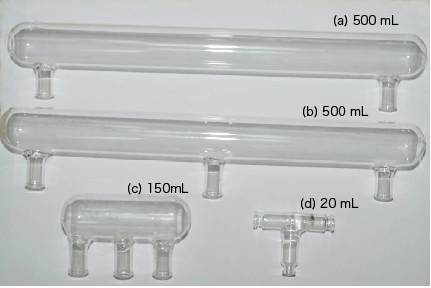
The equipment showed in the figure 1d was built starting from three small vials (5 mL). These small vials are liquemin ampoules or septum-capped medicinal vials, either new or used. In the case of used vials, they should be well washed and sterilized or disinfecting them in a pressure cooker. When these vials are ready, we asked the glass blower to prepare the T- Hofmann apparatus, the ampoules' bottom were cut out and then connected back to back in a T-shape.
The iron or platinum or silver electrodes were stick at the
T- Hofmann tubes from two sides through rubber stoppers; these
electrodes were connected by means of crocodiles to the AC-power
(see above). A small balloon was fixed on top of the T hand of
the apparatus.
Hazards
Although power sources used are relatively weak, electrodes
should not be handled while cells are operating. Care should be
exercised when testing the hydrogen gas with a burning splint,
be careful of explosive hydrogen and oxygen mixtures. It is a
good idea to burn the gases off to prevent a dangerous mixture.
Handle the acid and base and Sodium Sulfate with care; it cause
skin irritation. Wear gloves and goggles throughout the experiments,
or wear any eye protection. It is the responsibility of teachers
doing this demonstration to carry out an appropriate risk assessment.
Reagents
Na2SO4 (100%),
H2SO4 (96-98 %),
NaOH (100%).
a). Preparations previous to the electrolysis.
Preparation of the electrolyte and the test solutions:
1M Na2SO4 Solution
1M H2SO4 Solution
1M NaOH Solution
b). Electrolysis experiments.
When an electrical current (DC) is passed through a sodium
sulfate, oxygen gas and hydrogen ions H+ are
produced at the anode while hydrogen gas and hydroxide ions are
released at the cathode side; see the equations (4-7):
Cathodic reaction: 4 H2O + 4 e-
![]() 4 OH-(aq)
+ 2 H2(g)
4 OH-(aq)
+ 2 H2(g)
Anodic reaction: 2 H2O ![]() 4 H+(aq) + O2(g) + 4 e-
4 H+(aq) + O2(g) + 4 e-
Overall process: 6 H2O ![]() 2H2(g) + O2(g) + 4H+(aq) + 4OH-(aq)
2H2(g) + O2(g) + 4H+(aq) + 4OH-(aq)
Red cabbage contains pigments call anthocyanins. Anthocyanins belong to group of chemical compounds called flavonoids. This pigment is found in many flowers, fruits and fall leaves, and is responsible for many of the reds, blues, and purples you see around you. It makes cornflowers blue, pumpkins orange, strawberries red, and cabbage purple. For most pH indicators, the compound acquires a proton at low pH (lots of H+) but looses it at higher pH. This seemingly minor alteration is sufficient to alter the wavelengths of light reflected by the compound, thus creating the color change with respect to pH. Anthocyanins behave somewhat inversely in that the pigments "gain" an -OH at basic pH, but loose it at acidic pH (3). The plant pigments present in red cabbage (anthocyanins), turn to red in acidic solutions, purple in neutral solutions, and green to yellow in basic solutions (4-7).
It can be clearly observed after a few minutes of electrolysis
that the color of the indicator turns as the following (7):
Anode: purple ![]() red
red
Cathode: purple ![]() green
green
The intensity of the color is dependent on the change in the pH of the solution near the electrode. As a result of electrolysis of water, bubbles of hydrogen gas as well as hydroxide ions accumulate in the cathode side. On the other hand, bubbles of oxygen gas and also hydrogen ions are produced in the anode side. Therefore, after a few minutes from closing the electric circuit it can be clearly observed that the color of the indicator turns into green near the cathode, while it change to red at the anode (7).
(a) (b)
(c) (d)
Fig.2 shows T-apparatus which contains 1M Na2SO4 solution with red cabbage indicator. A small balloon was fixed on top of the T hand of the apparatus. The Pt electrode instead of using Pt electrode you can use Ni or Ag electrode) in the lower side of the apparatus were connected to an electrical source of 12VDC. Immediately after closing the electric circuit, a quick change in the color of the solution occurred: green color on the cathode denoting the formation of basic environment, and red color on the anode indicating the formation of acidic environment and the gas bubbles, oxygen at the anodic reaction and hydrogen at the cathodic reaction are collected on the balloon (fig 2). When the balloon is full with gases (Oxygen and Hydrogen), it is possible to take it over and tide it carefully. In a safe area, the balloon will be igniting to corroborate in a conventional way the presence of hydrogen and oxygen, care should be exercised when testing the hydrogen gas with a burning splint (see Hazard). When placing the balloon carefully on a fire source, a strong sound of bomb will be produced (the hydrogen contribution) and a bright flame (the oxygen contribution)
2H2 (g) + O2 (g) 2H2O (l)
When we exchange the platinum or nickel anode by Fe electrode (hypodermic needles, 0.80 x 40 mm) (see Fig. 3), we observed the same results as in the previous experiment, such as the colour changes produced by the indicator in the cathode, but no colour change and no gas is observed at the positive electrode (anode) and one can observe rusty solution when the experiment is over, see the equations:
Reduction reaction: 2 H2O(l)
+ 2 e- ![]() H2(g) + 2 OH-(aq)
H2(g) + 2 OH-(aq)
Oxidation reaction : Fe(s) ![]() Fe2+(aq)
+ 2e-
Fe2+(aq)
+ 2e-
Overall reaction: 2 H2O(l)
+ Fe(s) ![]() Fe2+(aq) + 2 OH-(aq) + H2(g)
Fe2+(aq) + 2 OH-(aq) + H2(g)
Fe2+(aq)
+ 2 OH-(aq) ![]() Fe(OH)2(s)
Fe(OH)2(s)
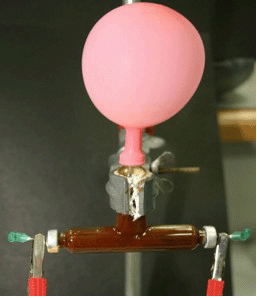
Fig. 3: Electrolysis of water solution in T- Hofmann apparatus
with Fe-Electrodes.
The iron metal takes less energy, so it happens more easily
than the water splitting reaction in the anode. Nichrome wire
also works, and it's quite a bit cheaper than platinum (you can
get it at a hobby store).
By adding drops of diluted sulfuric acid this precipitate disappears:
Fe (OH)2(s)
+ 2 H+ (aq) ![]() Fe2+(aq) +
2H2O
Fe2+(aq) +
2H2O
Drops of 0.1 M KMnO4 solution lose their purple colours because we produce Mn2+(aq) which colourless, showing that Fe2+ ions were present:
5 Fe2+(aq)
+ MnO4-(aq)
+ 8 H+(aq) ![]() 5
Fe3+(aq)
+ Mn2+(aq) + 4 H2O
5
Fe3+(aq)
+ Mn2+(aq) + 4 H2O
In order to test for the presence of iron (Fe3+)
ions, some drops of the solution taken from the positive electrode,
are transferred into two other cavities of a blister (sample 1
and sample 2). One drop of potassium hexacyanoferrate (Fe2+) solution is added to sample 1: A blue
precipitate is observed indicating that the solution contains
iron (Fe3+) ions. On the sample
2, one drop of ammonium thiocyanate solution is added, A red brown
colour appears, indicating that the solution contains iron (Fe3+) ions. These tests prove that we have
(Fe2+) in the solution which
produced through the electrolysis.
Conclusions
Using T- Hofmann apparatus which built from disposable materials for electrolysis processes allow students to visualize what happens during electrolysis. The inexpensive and simple apparatus introduced here make these experiments safely available. Students have conducted these experiments in our classes and find them fun and exciting, especially when they visually observing the color changes of the indicator near the electrodes, and collecting and burning the gases. Although they believe that using this simple and inexpensive apparatus makes the experiments simpler, it also make them think about obtaining alternative materials if they cannot use laboratory equipment or materials.
1. Zhou, R. E., J. Chem. Educ.,
73, 786 (1996).
2. Hendricks, L.J. and Williams, J.T.,
J. Chem. Educ., 59, 586 (1982).
3. Shakhashiri, B. Z. "Chemical
Demonstrations: a Handbook for Teachers of Chemistry",
Vol. 4, p. 156 (1992).
4. Hugerat, M., Basheer, S. and Schwarz,
P., The Chem. Educator, J., 9 (1), 24-26 (2004).
5. Hugerat, M. "New Prospects in Teaching Microscale Electrolysis"
Chapter in Microscale Chemistry Experimentation for
all Ages, Hugerat, Schwarz, Livneh (Eds.), pp. 141-155 (2006).
6. Hugerat, M. "Less is more! In Teaching Chemistry, All
You Need is One Drop!" Chem. Educ. J., 9 (2),
No. 13 (2006), http://chem.sci.utsunomiya-u.ac.jp/v9n2/MHugerat/MHugerat.html.
7. Ning-Huai Zhou, W. Habelitz-Tkotz,
D. Giesler, M.K. El-Marsafy, P. Schwarz, M. Hugerat, M. Najdoski,
"Quantitative Microscale Chemistry Experimentation",
J. Sci. Educ., 6 (2), 84-88 (2005).