Chemical Education Journal (CEJ),
Vol. 11, No. 2 /Registration No. 11- 10 /Received March 26, 2008.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Modern Teaching Materials
Basic meaning of modern teaching materials used in general
chemistry teaching is to place students into situations where
they can learn and acquire, inquire and obtain knowledge. This
teaching approach may have the positive influence on the development
of students' thinking, attitudes and active participation in the
process of learning. Similarly, it stimulates students' interactions
through dialogues and disscusions, developing skills in handling
and using technical means (1,2),
and in ambiguous learning of chemistry, computer science and foreign
languages. In that respect, teaching materials have to be obvious,
interesting and easy to handle in presenting teaching content.
Teaching materials have to be mediators between scientific knowledge
and students for easy covering of teaching content.
The computer is a powerful means of mass communication (3). In addition to providing information and entertainments, it can also play a major role in education. We should bear in mind, however, that the computer is just one of the available teaching materials and not the only one. The video materials are certain to stimulate students' curiosity and help them learn. The best results are achieved when it is used together with other teaching materials.
Virtual Chemistry Lab
Virtual Chemistry Lab is software, aiming at illustration
and brief demonstration of those experiments, which are unable
to present to students in any other way. It is used for simulation
of experimental procedures and it allows students to see the procedures
by proposing certain solutions and monitoring the results. It
is one of the teaching materials defined as didactically shaped
reality (3).
Owing to specific and various possibilities, Virtual Chemistry Lab presents the experimental procedures in modern, clear and interesting way.
Students have to be prepared for active participation in the realization of our curriculum. They have to be acquainted with the purpose of this software, which will arouse their interest and allow us to set the observation tasks (i.e., highlight the key for moments, which deserve their undivided attention). Before we start the software, we can revise with students some of the previously covered material necessary for them to fully understand the software. The aim of these activities is to enable students to comprehend the experiments featured in the software in better way and gradually. The information acquired in this way has to be made concrete and allowed to set firmly in the minds of the students by series of questions prepared in advance. The acquired knowledge is generalized, the concepts are integrated into a system and the gained information may be revised (4).
Pre-lab activity
Titration Technique is a laser videodisc that includes 20
lessons and demonstrations of laboratory techniques related to
titration. It is suitable for using in introductory chemistry
classes at high schools or colleges. As far as possible, the techniques
are shown in a close-up and full-screen manner, focusing attention
on a "single point lesson". Each video-scene has been
selected because it illustrates an important aspect of the laboratory
technique that can be shown to students prior to titrations in
the chemistry laboratory. Showing this video to students as a
pre-lab activity will help make their limited time in the laboratory
more effective and safe.
Methodology
Figure 1 shows the virtual laboratory. The panel on the left
is a customizable stockroom of chemical reagents, which may include
either common reagents and/or chosen materials for which parameters
are specified by the instructor. The middle workspace provides
an area for performing experiments. The panel on the right supplies
multiple representations of the properties of the selected solution,
including temperature, pH, and a list of given chemical species
with amounts shown in mol, grams or concentration. These quantities
are the variables in the computational procedures of the course,
and so this panel provides the simulation of the traditional lab
experiment.
Classroom observations, involving 30-35 students working alone
or as pairs, have been used to gain insight into students' interactions
with various types of activities. These observations have informed
the design of our activities and have allowed us to formulate
targets to be addressed by more controlled experiments.
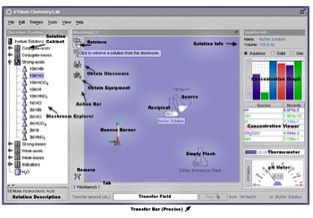
Figure 1. Interface of the virtual lab for acid-base titrations.
The panel on the left is the customizable stockroom of chemical
reagents and/or chosen materials. The panel on the right shows
multiple representations of the contents of the selected solution.
Acid-base titrations
Acid-base titrations are based on reactions between acids
and bases, which are sometimes called by their traditional name
- "neutralization". However, the term "neutralization"
is not quite suitable, because neutral environment is the result
solely in the case of interaction between aqueous solutions of
strong acids and strong bases. The protolytic reaction can be
represented as:
H3O+ + OH- <=> 2H2O (1)
This means that pH value will be 7 when equimolecular amounts
of strong monobasic acids and strong monoacidic bases interact:
HA + BOH -> B+A-
+ H2O (2)
Based on this fact, volumetric neutralization methods have
been developed, and they are used for determining unknown concentrations
of acids or bases. They can be alkali-metric (if titration is
done with a base) or acidi-metric (if titration is done with an
acid).
Choice of Lab Equipment
From a list of equipments available, students can choose equipments
necessary for this experiment:
- an Erlenmeyer flask (100 ml)
- a beaker (100 ml)
- a pipette
- a burette (50 ml).
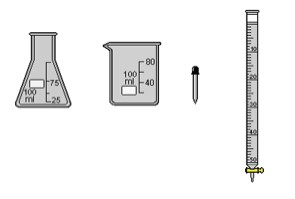
Figure 2. Lab equipments
Virtual Chemistry Lab Software Procedure
To determine the concentration of HCl using the alkali-metric
method the 0.2 M NaOH is used. The 0.2 M NaOH (a strong base)
is added into the HCl (a strong acid).
HCl + NaOH -> NaCl + H2O (3)
Titration Procedure
A 35 ml portion of the HCl solution is poured into the Erlenmeyer
flask and a suitable indicator (phenolphthalein) is added. Phenolphthalein
is used as the indicator; its color changes in the pH range 8.2
to 10.0. First, the pH of the solution is measured by means of
a pH-meter. Then, the burette is filled with the 0.2 M solution
of NaOH. Several drops of the NaOH solution are added into the
Erlenmeyer flask and it is shaken well. It is very important to
see the moment when the solution changes its color. That is the
titration equivalence point, i.e., the point of interaction between
the equal amounts of the acid and the base. At that point titration
is completed and the volume of the NaOH solution used is recorded.
This data is used for calculating the unknown concentration of
the HCl.

Figure 3. Titration procedure
The performed students' activities could be determined as follows:
1. Calculate and check. After a traditional paper-and-pencil problem-solving activity (5), students perform an experiment to verify their results. The educational goal is to have the students examine their calculation after it is complete. Using the virtual laboratory to check the answers instead of looking them up in the back of a textbook requires a thoughtful assessment of the relationship between the computation and the authentic chemistry experiment. Our observations suggest that this shift from mathematical problem-solving to the experiment is a nontrivial step requiring careful consideration of computations.
2. Online experiments. Students are given a goal and the lab is equipped with various chemical solutions, equipment, and solution viewers. This is similar to the setting up of a physical lab and many of our activities are indeed parallel to what would be done in a physical lab. However, because experiments can be done quickly and safely, students can be given greater flexibility in the design of their experiment.
3. Layered activities. Here, students perform a set of activities involving the same chemical system, but modeling the system with varying levels of complexity and approximation. The approximations can either be removed or invoked as one moves through a series of problems. These interconnected layers, particularly with the addition of structured debriefing, invite students to reflect on how the removal or addition of an assumption changes both their problem-solving approach and the predicted results.
Sisovic and Bojovic (6) suggest two cooperative learning forms for the teaching concept of topic "acids and bases" in the first grade of the ordinary secondary school. This cooperative approach comprises students'-group and "teacher-student" forms. The results of statistically processed final tests show that students, who learned in the experimental group, by the suggested cooperative approach, show 16% higher points at the level of reproduction, 22% at the level of understanding and 14% at the level of application. However, although these observations are encouraging, some students have judged this approach as intruding too much experimental work and students thinking.
As a variation of this approach, we suggest computer supported approach for teaching the concept of the topic "acids and bases". It comprises the following forms: teacher-student and students work individually/in groups using computers. We strongly believe that this concept offers the solution for the students' judgment of too much experimental work, but at the same time infallibly comprises students' active learning and thinking. This approach may be a key for economical rationalization of avoiding the use of laboratory equipment and substances, when they are not so infallible. It gives the clue for the contemporary problem, when time is money, teaching to be more efficient and easier. The authors believe that this approach put students in the situation of learning ambiguous, according to modern standards.
Many researches have referred to computer supported collaborative learning (7-10), trying to find empirical evidence for the benefits of this learning concept. Although they showed some improvement in learning and achievement (11), still other research shows that not all students have benefit from the collaborative experience (12). It is worth underlining the thought (J. Trefil) (13) who stressed the benefits of traditional chalk-and-blackboard teaching style. Namely, he quoted that the novel presentation media (ex. power point presentation) cannot compensate the poor quality of the lecture material and that other media may be successfully used for teaching as well. These statements are similar to our findings that computer supported teaching is an additional approach to the traditional teaching style only when it is used as an additional approach to the traditional one. Our suggestion is not aiming at exclusion of laboratories, manual laboratory work and students' pencil-and-pen learning.
Taking in mind the obvious advantages and disadvantages of this approach, we felt conscious that our research may be the direction of further development or supplementation of educational process. In our opinion, these designed teaching concepts will introduce cooperative and computer supported teaching or their interplay in different extents depending on the topic concerned. The experimental results obtained by other authors and our observations gave us the opportunity to underline advantages and disadvantages of using computer supported teaching approach in comparison with traditional "teacher-student" lecture approaches.
Advantages of Virtual Chemistry Lab Software
This is a list of advantages in comparison with the traditional
laboratory work performed by students, individually or in groups:
- teaching improvements
- the economical aspect
Disadvantages of Virtual Chemistry Lab Software
Here is a list of disadvantages in comparison with the traditional
laboratory work performed by students, individually or in groups:
- teaching improvements
- the economical aspect
Virtual Chemistry Lab software can be used for completing various educational tasks in chemistry teaching, such as:
We should bear in mind that the best educational effects are achieved if this teaching tool is used in combination with other teaching tools, since it serves as an excellent addition to work in chemistry laboratories. Scientific and technological advancement requires content and rationalization of both contents and organization in all aspects of education. Our conclusion is that the goal of the virtual lab is not to replace the physical laboratory, but rather, it is to help students connecting their paper-and-pencil work to actual chemical problems.
Corresponding author: PhD Danijela Kostic, Associate Professor; Faculty of Natural Sciences and Mathematics, University of Nis, Visegradska 33, 18000 Nis, Serbia;
Literature Cited
1. Francisco, J.; Nicoll, G.; Trautmann,
M. J. Chem. Educ. 1998 75 210.
2. Kovac, J. J. Chem. Educ. 1999
76 120.
3. Alessi, S.; Trollip, S. Computer-based
instruction: Methods and development, Prentice Hall, Inc.,
New Jersey, 1991.
4. Hanson, D.; Wolfskill, T. J.
Chem. Educ. 1998 75 143.
5. Browne, L.; Blackburn, E. J.
Chem. Educ. 1999 76 1104.
6. Sisovic, D.; Bojovic, S. Chem.
Educ.: Resear. Pract. Europ. 2000 1 (2) 263.
7. Cohen, A.; Scardamalia, M. Interact.
Learn. Environ. 1998 6 99.
8. Hoadley, C.; Linn, M. J. Sci.
Teach. 2000 22 839.
9. Lipponen, L. Learn. Environ.
Resear. 2000 3 179.
10. Lipponen, L.; Rahikainen, M.; Lallimo,
J.; Hakkarainen, K. Learn. Instruct. 2003 13
487.
11. Eilks, I. J. Chem. Educ.
2005 82(2) 313.
12. Webb, N.; Mastergeorge, A. Internat.
J. Educ. Resear. 2003 39 73.
13. Trefil, J. J. Bullet. Chem.
Techn. Maced. 2004 23 (2) 193.
14. Dori, D.; Dori, Y. J. Comput.
Math. Sci. Teach. 1996 15 (1/2) 6584.
15. Smith, S.; Jones, L. J. Chem.
Educ. 1989 66 (1) 8.
16. Yal nalp, S.; Geban, Ö.;
kan. J. Resear. Sci. Teach. 1995 32 (10)
1083.
17. Ramos, M; Fernandes, P. J. Chem.
Educ. 2005 82 1021.
Figures captions
Figure 1. Interface of the virtual lab for acid-base titrations. The panel on the left is the customizable stockroom of chemical reagents and/or chosen materials. The panel on the right shows multiple representations of the contents of the selected solution.
Figure 2. Lab equipments
Figure 3. Titration procedure

Figure 1.

Figure 2.

Figure 3.