Chemical Education Journal (CEJ), Vol. 11, No. 2 /Registration
No. 11-8 /Received January 23, 2008
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
*E-mail: <aronper![]() siu.buap.mx>
siu.buap.mx>
Keywords: Copper aspirinate, water electrolysis, Cu(OH)2, single crystals synthesis, aspirin.
Tetrakis-m-(acetylsalicylato)dicopper(II) (or copper aspirinate for short) is a very interesting biologically active compound that can be easily obtained starting from an aspirin tablet, a cup of drinking water and a piece of copper wire. Since in this experiment the quality of the single crystals was highly dependent on the concentration of the reactants (acetylsalicylic acid and copper hydroxide), a slow diffusion process could be achieved by the in-situ electrolytic generation of Cu(OH)2, followed by addition of a whole tablet of aspirin.
The experiment is quite simple and inexpensive as to be used for research or educational purposes and it affords high quality single crystals good enough to realize physical studies such as electron spin resonance, X-ray structural determination, etc.
Although aspirin, 2-(acetyloxy)benzoic acid or acetylsalicylic acid (Figure 1), is more than one hundred years old, it is one of the most common over-the-counter medicines in the world (Rinsema, 1999). In fact, since its industrial production by Bayer Company in 1899, it has dominated the market of inexpensive pain killers. Nowadays, 40 % of its human consumption is as cardiovascular protector, for it is effective even in the case of administration at low doses (Shen, 1997; Selke, 1997; Weiping, 1998; Yang, 2001; Aspirin, 2007).
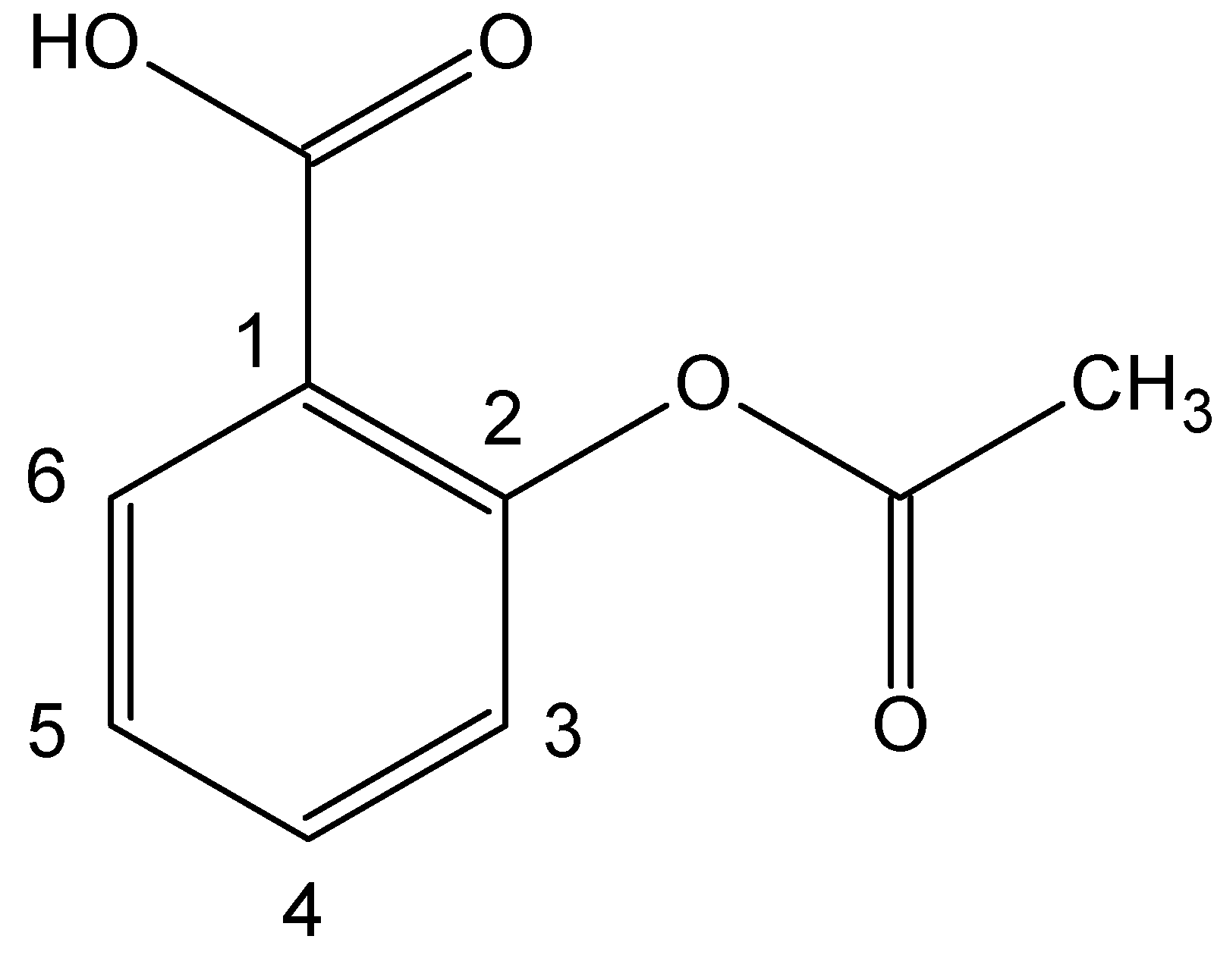
Aspirin possesses a number of properties that makes it one of the most often recommended drugs. It is an analgesic, an antipyretic and it is very effective in pain relief. It is also an anti-inflammatory agent, providing some relief from the swelling associated with arthritis and minor injuries. In contrast, it is not as innocuous as one might imagine from its widespread use and ready availability. In some persons its repeated usage may cause gastrointestinal bleeding, and overdoses can provoke a variety of reactions such as vomiting, diarrhea, vertigo and hallucinations. The recommended adult dose can vary from 0.08-1 g/6 h, while a single dose of 10-30 g can be fatal (LD50 orally in rats is 200 mg/kg).
As if these properties were not enough, new or increased biological and physical properties have been discovered when aspirin (or aspirinato anion) is coordinated to a transition metal. For example, the title compound is now recognized as a potential therapeutically useful drug due to its anti-inflammatory, antiulcer, antiischemic, anticancer, antimutagenic, anticonvulsant and antimicrobial activities (Sorenson, 1976; Chohan, 2002; Hsu, 2002; Fujimori, 2005). Additionally, the ternary complex Cu2(aspirinate)4(DMF)2 has been recently synthesized and its anticonvulsant activity was reported (Viossat, 2003; Fujimori, 2005).
On the other hand, very interesting physical properties are suspected in Cu2(aspirinate)4 because of its extended metal-organic framework nature and the strong Cu-Cu antiferromagnetic interaction observed in the solid state (Meier, 1985; Viossat, 2003). Thus, there is an increased interest for searching new techniques of synthesis of the title and related compounds, taking into account their potential interest in pharmacology and material science (Kim, 2001; Abuhijleh, 2001; Forster, 2002).
Copper(II) aspirinate was initially characterized over 35 years ago (Manojlovic-Muir, 1973). However, its crystal structure was recently re-determined in more detail (García, 2003). The usual technique of synthesis of copper-aspirinate (and other metallic carboxylates) is the classical methatesis between a salt containing the desired metal (i.e. chloride, nitrate, acetate or sulphate) and the neutral ligand or an alkaline salt of the ligand (Sorenson, 1976; Dudek, 1977; Borer, 2000; Fujimori, 2005) and only few efforts have been made in other directions, such as the typical acid-base reaction between copper(II) hydroxide and acetylsalycilic acid reported here.
Thus, the increasing importance of the copper-aspirin complexes in several fields of science explains the interest of some teachers in including the synthesis of this compound as a part of their undergraduate laboratory chemistry courses (i. e. Torre, 2001; Fraser, 2003); however most of the synthetic procedures only afford the blue amorphous or microcrystalline powder. In contrast, the synthetic procedure presented here yields nice single crystals of copper aspirinate suitable for physical measurements.
Although copper(II) hydroxide can be purchased or prepared from metallic copper (as a part of the "copper cycle": Todd, 1985), in this experiment it is generated by electrolysis of drinking water by using a copper wire as the sacrificial electrode. In this manner, four educational purposes can be achieved:
1. To exemplify a green reaction avoiding the use of strong acids in the synthesis of the copper supply (Cu(OH)2 or the salts of copper(II) mentioned above).
2. To tackle the synthesis of a very interesting coordination compound by means of a Chem Com approach because a very common materials are used in the reaction (an aspirin tablet, drinking water, house used electric copper wire and a cellular phone charger).
3. To illustrate that the slow diffusion process of the reactants (copper(II) hydroxide and acetylsalicylic acid) is a useful way to achieve single crystals suitable for physical measurements (e.g. X-ray structure determination).
4. A nice discovery-oriented experiment that can be used in a bioinorganic chemistry laboratory (as it is used in our case) or in other courses such as general chemistry, inorganic chemistry or electrochemistry laboratories.
Electrochemical processes are usually carried out under well
controlled reaction conditions, such as:
a). High purity of the solvent and reactants (Sakura,
1993).
b). A selected electrolyte to conduct the electrical current (Sometimes the electrolyte also contributes with the counter ion of the electroactive substance).
c). An electrochemical cell with separated anodic and cathodic compartments;
d). Expensive electrodes (e.g. platinum wires) which usually need a special pre-treatment.
e). A good control of the applied electrical current intensity or voltage which requires the use of sophisticated equipment (potentiostate or galvanostate).
f). And sometimes also a good control of the temperature (Penicaud, 1993).
In contrast, the electrochemical experiment here presented is carried out by using drinking water (which already contains enough electrolytes to conduct the electrical current), a piece of common copper wire which is used as sacrificial anode, a pencil carbon electrode as cathode, an AC eliminator or a cellular phone battery charger as a power supply, a disposable plastic container as an open electrochemical cell and a whole Bayer's aspirin tablet (500 mg).
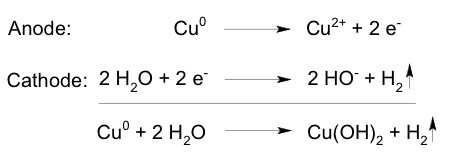
The electrochemical synthesis of copper(II) hydroxide by electrolysis of water using copper as sacrificial electrode is:
4. Chemical reaction
While the copper(II) hydroxide is being produced, the acetyl
salicylic acid contained in the aspirin tablet is slowly dissolved
by water and diffuses into the electrolytic cell. So, it is possible
to observe an interfacial phenomenon in which the acid-base reaction
occurs to yield single crystals of copper aspirinate at room temperature.
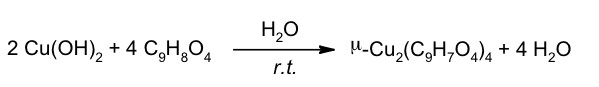
The equation of the reaction is:
A Bayer's aspirin tablet (ASPIRIN®, 500 mg) was carefully dropped near the wall of a jelly cup containing 60-80 mL of drinking water. An electrical device made of a potentiostate (a cellular phone charger, a battery eliminator or a commercial 3-9 volt battery), a carbon pencil lead (0.7 mm diameter) as cathode electrode, a piece of copper wire (1-2 mm diameter) as anode electrode and a pair of alligator clips that completed the experimental setting (Figure 2). In accordance with the electrochemical reaction described above, when the circuit was closed, a great quantity of small H2 bubbles were produced at the carbon electrode, while around the copper electrode the solution turned cloudy in a few minutes, with the characteristic pale blue color due to the formation of Cu(OH)2. Otherwise, the polarity of the current supply was inverted.
| |
|
|
|
|
Working at 6 volts, the current was suppressed after 6 h of electrolysis and the mixture was let stand at ambient conditions to complete the acid/base reaction. After ca. 48 h, hexagonal prismatic turquoise blue single crystals (Figure 3) of tetrakis-m-(acetylsalicylato)dicopper(II) were picked out by using a Pasteur's pipette.
Because of the slow diffusion of the reactant species during the reaction process, both an electrocrystallization process (crystal growth of copper-aspirinate over the copper electrode) and some unidentified intermediate species due to the acid/base reaction was also observed at the interface.

In spite of the simplicity of this reaction, it encloses some interesting details. For instance, we found that high concentration of acetylsalicylic acid at the beginning of the reaction promotes the formation of a green solution (probably copper-salicylate) instead of the turquoise blue single crystals of copper-aspirinate. This fact prompts us to carry out the experiment by using the whole tablet (see experimental part). Although, in this reaction the tablet's excipient (acetylcellulose) appears as a contaminant of the product, several single crystals of sufficient crystallographic quality could be separated from the mixture and checked on a single crystal X-ray diffractometer by a group of our collaborators (Bouhmaidal, 2005) and also by ourselves. In both cases the cell parameters of the crystals were identical to those reported in the literature (García, 2003).
Generic tablets of acetylsalicylic acid also did not work very well probably due to a difference in the velocity of tablet's solubility (generic tablet disintegrated faster than Bayer's aspirin tablet). On the other hand, the electrolytic production of copper(II) hydroxide was very slow when distillated water was used and the results were not good, so in view of an inexpensive experiment, commercial sodium chloride was added (12 mg/80 mL water) as electrolyte support to afford a result similar to that obtained by using drinking water.
7. Internal and external glance of a single copper aspirinate crystal
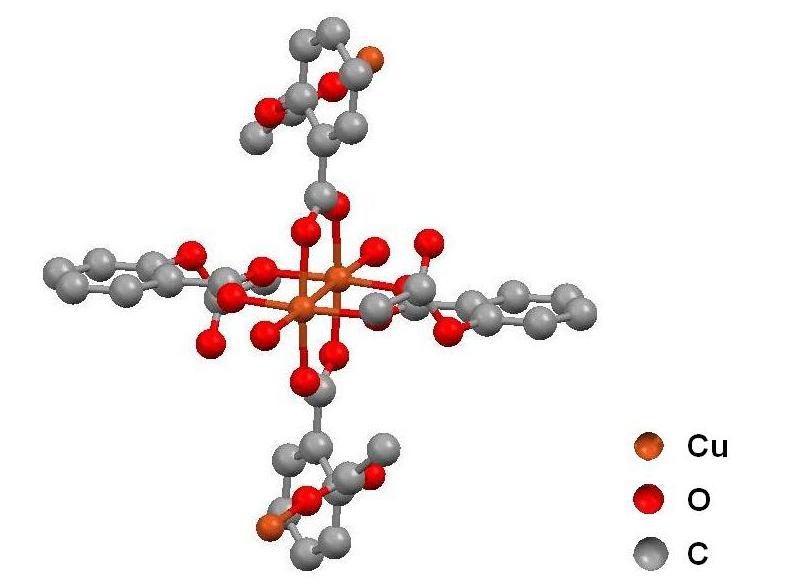
The visualization of the crystal structure of copper aspirinate is a good opportunity for encouraging students to learn and use computational programs aimed at analysis of the architecture of crystal structures. For example, we used the Mercury 1.4.2 program (Mercury, 2008) for reading and manipulating the structural data of the title compound that are available as a cif-file in the web site of the International Union of Crystallography (IUCr, 2008). Both can be downloaded free of charge via internet.
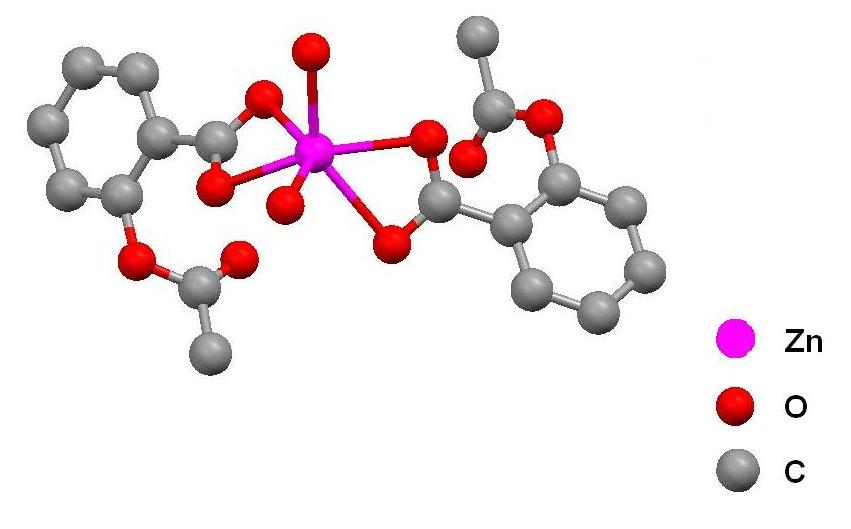
After a short introduction about the Mercury's tools or the reading of its tutorial (Daron, 2005), our students were able to analyze the X-ray structure of copper aspirinate (Figure 4a) in different display styles, and measure its geometric parameters such as the Cu(II)-Cu(II) distance. This close contact is related to the antiferromagnetic behavior of their d9-d9 unpaired electrons, which is typical of the º-bridged coordination compounds formed between carboxylate and copper(II) ions (Melnik, 1981; Melnik, 1982). In addition, the crystal structure of copper aspirinate can be compared with the structure of other metal aspirinates such as cadmium aspirinate (Viossat, 2003; Vázquez Arciga, 2004). In this case, we encourage our students to observe the binuclear nature of the copper compound vs the mononuclear feature of the second one (Figure 4b).
Moreover, in case a powder diffractometer is locally available, an experimental powder pattern can be measured on a sample of copper(II) aspirinate and compared with that generated by Mercury for assessing the purity of the material synthesized as mentioned above.
 |
 |
|
|
|
On the other hand, the single crystals obtained by the students in their experiments may be seen under the microscope and their photographs were taken. We encouraged our students for making a competition of the best picture of their crystals, based on the criteria that large crystals possessing well defined faces are qualitatively better. The dimensions of the crystals obtained by the students ranged from 0.3 to 0.8 mm from one vertex to the opposite one (see Figure 3 as an example).
An inexpensive experiment which illustrates the preparation of a pharmacologically relevant compound via two consecutive reactions (electrochemical-chemical) was achieved. The quality of the single crystals obtained by this method is amazing when the crude conditions of the synthesis were considered. The experiment is so simple but at the same time complete as to be used from high school to undergraduate levels, depending on the purposes of the teacher.
Our students were very motivated when they could reach both internal and external glances of their copper aspirinate crystals and especially so when a character of scientific research was included in a subsequent sessions in which they probed the biological activity of copper and cadmium aspirinates in biological systems (Palacios-Hernández, 2004).
Abuhijleh L., Woods. C. (2001).
Inorganic Chemistry Communications. 4 (3),
119-123.
Aspirin (2007). "The world of ASPIRIN®".
On-line: <http://www.aspirin.com/world_of_aspirin_en.html#>.
Last visited on Jan. 21, 2008.
Borer L. L., Barry E. (2000). J.
Chem. Educ. 77, 354.
Bouhmaidal N., Fraisse B., Pérez-Benítez
A., Méndez-Rojas M. A., Ghermani N. E. (2005). Acta
Cryst. A61, C311.
Chohan Z. H., Iqbal M. S., Iqbal H.
S., Scozzafava A, Supuran C. T. (2002). J. Enzyme. Inhib. Med.
Chem. 17 (2), 87-91.
Daron E. J., Gregory J. G. (2005).
"Integration of X-ray Crystallography into the Undergraduate
Curriculum Using Mercury© Software". 57th
Southeast/61st ACS Southwest Regional Meeting.
Dudek E. (1977). J. Chem. Educ.
54, 329.
Fraser, B. (2003)
"Copper Aspirinate Synthesis". On-line: <http://www.geocities.com/scripturalphysics/etc/CopperAspirinate.html>.
Last visited on Nov. 17, 2008.
Forster P.M., Thomas P.M., Cheetham,
A. K. (2002). Chem. Mater. 14, 17-70.
Fujimori T., Yamada S., Yasui H., Sakurai
H., In Y., Ishida T. (2005). J. Biol. Inorg. Chem.
10 (8), 831-841.
García
F., Méndez-Rojas M. A., González-Vergara E., Bernès
S., Quiroz M. A. (2003). Acta Cryst. E59, m1171-m1173.
Hsu C. S., Li Y. (2002). Biochem.
Biophys. Res. Commun. 293 (2), 705-709.
IUCr (2008). "Crystallography
Journals Online - paper details. Online", <http://www.iucr.org/cgi-bin/paper?hg6010>.
Last visited on Jan. 21, 2008.
Kim J., Chen, B., Reineke T. M., Li
H., Eddaoudi M., Moler D. B., O'Keeffe M., Yaghi O. M. (2001).
J. Am. Chem. Soc. 123, 8239-8247.
Manojlovic-Muir L. (1973). Acta
Cryst. B29, 2033-2037.
Meier J. L., Coughenour C. E., Carlisle
J. A., Carlisle G. O. (1985). Inorg. Chim. Acta. 106,
159.
Melnik M. (1981). Coord. Chem. Rev.
36, 1-44.
Melnik M. (1982). Coord. Chem. Rev.
42, 259-293.
Mercury - Download -. (2008). Online
<http://www.ccdc.cam.ac.uk/free_services/mercury/downloads/>.
Last visited on Jan. 21, 2008.
Palacios Hernández T., Moreno
Martínez A., Ramírez Rodríguez J., Calderón
Morales L., Pérez-Benítez A., Vázquez
Arciga H., Méndez-Rojas M. Á., y Rivera Tapia J.
A. (2004). "Evaluación farmacológica de acetilsalicilato
de cadmio [Cd(C9H7O4)2.2(H2O)] y CdCl2 en conejos con artritis inducida por mycoplasma
fermentans p-140". XXXVII Congreso Nacional de Ciencias Farmacéuticas
2004. Acapulco, Gro. México. Unpublished results.
Penicaud A., Pérez-Benítez
A., Gleason V. R., Muñoz P. E., Escudero R. (1993). J.
Amer. Chem. Soc. 115, 10392-3.
Rinsema T. J. (1999). Medical History.
43, 502-507.
Sakura S., Anzai H. (1993). Electrochem.
Acta. 38 (15), 2343-2350.
Sellke F. W., Richter H. W., Dunphy
G, Azodi M, Ely D. L. (1998). J. Surg. Res. 80 (2),
171-6.
Shen Z. Q, Chen Z. H., Ma G. Y., Wang
D. C., Wu W. L., Liu W. P., Yang Y. K., Xiong H. Z. (1997). Zhongguo
Yao Li Xue Bao. 18 (4), 358-62.
Sorenson
J. R. J. (1976). J. Med. Chem. 19 (1), 135.
Todd D., Hobey W. D. (1985). J.
Chem. Educ. 62, 177.
Torre M. H., Facchin, G. (2001). "Síntesis
de aspirinato de Cu(II)". Online < http://icur.edu.uy/Investigadores/PEDECIBA/TorreMariaHpubli.htm
>. Last visited on Jan. 21, 2008.
Viossat
B., Daran J. C., Savouret G., Morgant G., Greenaway F. T., Dung
N. H., Pham-Tran V. A., Sorenson J. R. (2003). J. Inorg. Biochem.
96 (2-3), 375-85.
Weiping L, Huizhou X, Yikun Y, Ling
L., Zhiqiang S., Zhihe C. (1998). Metal-Based Drugs. 5
(3), 123-126.
Yang W. M., Shen Z. Q., Chen Z. H.,
Li L., Peng F., Liu W. P. (2001). Acta Pharmacol. Sin.
22 (2), 121-124.