Chemical Education Journal (CEJ),
Vol. 12, No. 1 /Registration No. 12-5/Received August 6, 2008.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
E-mail: aronper![]() siu.buap.mx
siu.buap.mx
Keywords: Three-dimensional dynamic model, SN1 and SN2 mechanisms, Walden's inversion, racemization.
Contents
1. Abstract (In English).
2. Abstract (In Spanish).
3. Introduction.
4. Construction of the model.
5. Classical examples of reactions
that occur under the SN1 and SN2
mechanisms.
6. Conclusion.
7. References.
1. Abstract (In English)
A homemade model that allows illustrate the geometrical changes
occurred in a tetrahedral stereogenic center as a result of both
SN1 and SN2 mechanisms
is presented.
The model is based on a skeletal trigonal bipyramid that functions as a support of the tetrahedral reaction center and its substituents, either in the initial state, in the intermediate or transition states (depending on the type of mechanism) and in the final state. Because of the model is easy to make and very inexpensive, all of the students can construct their own model as a motivational workshop in the classroom or in the laboratory.
2. Abstract (In Spanish)
Se presenta un modelo dinamico tridimensional de facil construccion,
que permite ilustrar objetivamente los cambios geometricos que
ocurren durante los mecanismos de reaccion SN1
y SN2.
El modelo esta basado en una bipiramide trigonal que funciona como soporte del centro de reaccion y de sus sustituyentes. El mecanismo consiste en un simple equilibrio de fuerzas entre las ligas que simbolizan a los enlaces permanentes y los que se forman o se rompen con motivo de la reaccion de sustitucion.
El modelo es facil de construir y muy barato, por lo que cada estudiante podria elaborar su propio modelo en el salon de clase o en el laboratorio, lo cual, en nuestra experiencia, es una actividad que motiva mucho a los estudiantes.
3. Introduction
The teaching-learning of the mono- and bimolecular nucleophilic
substitution mechanisms (SN1 and SN2, respectively) is an obligated subject in
the basic organic chemistry courses (Chapter 4: SN1
mechanism, 2008; Chapter 4: SN2 mechanism, 2008;
Fox, 2004; Hornback,
2005; Wade, 2004). On the other
hand, in spite of the great advancements in the computational
software for illustrating these mechanisms (see for example: Berger, 2008a-b), some students experience
difficulties to visualize the geometrical changes that the reaction
center suffers because sometimes they are not capable to recognize
the geometrical forms. For example, in a test previous to a basic
organic chemistry course, a small group of our students drew two
tetrahedra sharing an apex instead of a trigonal bipyramid (Figure 1).
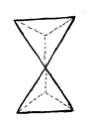
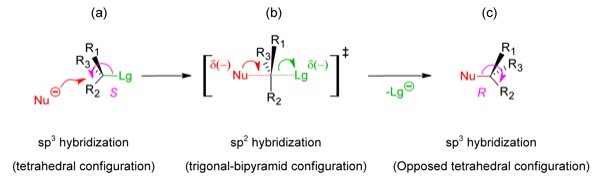
The understanding of the geometry is fundamental for the comprehension of the stereochemistry of these mechanisms. For example, in the SN2 mechanism (Figure 2), that is characterized by the participation of both the nucleophile and the substrate in the path that determines the velocity of the reaction (the formation of the transition state), the reaction center acquires a trigonal bipyramid configuration (Figure 2b).
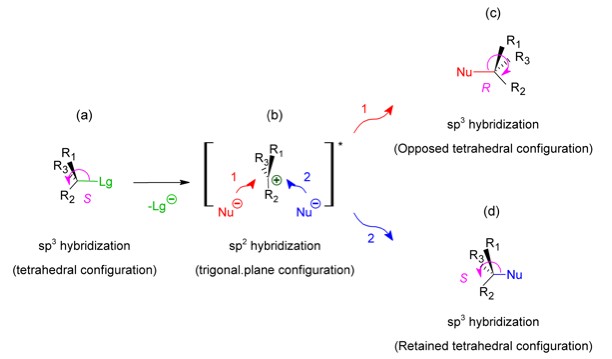
On the other side, the SN1 reaction mechanism is characterized by the slowness of dissociation of the substrate's C-Lg bond in the path that determines the velocity of the reaction: The formation of the intermediate state in which the reaction center assumes a trigonal plane configuration (Figure 3b).
Thus, if the reaction center is chiral in the initial states
of the SN2 and SN1
mechanisms, then its chirality in the final states will be respectively,
opposed (Walden's inversion, Figure 2c)
and both opposed and retained (Recemization process, Figures
3c and 3d).
So, if one whishes to build a physical model capable to illustrate
these reaction mechanisms, one needs to take in account the following
topological changes:
SN2 mechanism: Tetrahedral - trigonal bipyramid
- tetrahedral geometries.
SN1 mechanism: Tetrahedral - trigonal plane
- tetrahedral geometries.
In this manner, one of the following two strategies had been
used in the literature (Anderson, 1987;
Buist, 1991; Garret,
1978; Hamon, 1970; Newman,
1975; Noller, 1947; Nyquist,
1965; Sands, 1995; Sone,
1973), being the number one the most favored:
1. The construction of dynamic models possessing its mechanism
at "the central atom".
2. The construction of dynamic models possessing an external artifact
that shifts the "central atom's substituents" at the
required positions.
We followed the second choice for building our model, in which the external artifact is a trigonal bipyramid whose equatorial and apical vertices host respectively, to the permanent substituents (R1, R2 and R3 in the Figures 2 and 3) and the removable Lg and Nu groups. So, in our model the central atom is permanently linked to the equatorial vertices by means of rubbers and it is temporally joined to the apical vertices depending on the desired state that wants to be represented (Figure 4).
The operation of the model is really simple because the configuration
of the central atom is governed by the equilibrium between the
forces involved in a given reaction's path; it means, by the number
of rubbers linking the central atom with the vertices of the bipyramid:
a). Central atom bonded to three equatorial vertices: Trigonal
plane configuration (Figure 4b).
b). Central atom bonded to three equatorial vertices and one apical
vertex: Tetrahedral configuration (Figures
4a, c, d, e, and g).
c). Central atom bonded to three equatorial vertices and two apical
vertices: Trigonal bipyramid configuration (Figure
4f).
Once the trigonal bipyramid is constructed and the central
atom is permanently connected to the equatorial vertices, all
we have to do for illustrating the different reaction states is
connect the central atom to 0, 1 or 2 apical vertices. So, in
accordance with the Figures 2 and 3, the SN1 and SN2 reaction mechanisms are respectively illustrated
by using the present model in the Figures
4a-d and 4e-g.
|
|
|
|
|
|
|
|
|
|
At this point is necessary to leave in clear two aspects:
1. A trigonal bipyramid can be considered as two tetrahedra sharing
a face (the plane described by yellow straws in the model of the
Figure 4). If R1, R2,
and R3 are not equal to one another, that
face is a prochiral plane in the intermediate state of SN1 reaction or a chirality plane between the
two final states of the same reaction. This means that one tetrahedron
is an enantiomer of the other and vice versa.
2. These mechanisms are usually illustrated as the displacement of the substituents R1, R2 and R3 with respect to the reaction center. So, if the model is operated catching it by the external support then the relativity of the movement has to be considered because it appears as if the central atom is moving with respect to the substituents, it means inside of the bipyramid. This fact can be ignored or in order to be coincident with the textbooks' illustrations, the model must be handled by catching the central atom.
4. Construction of the model.
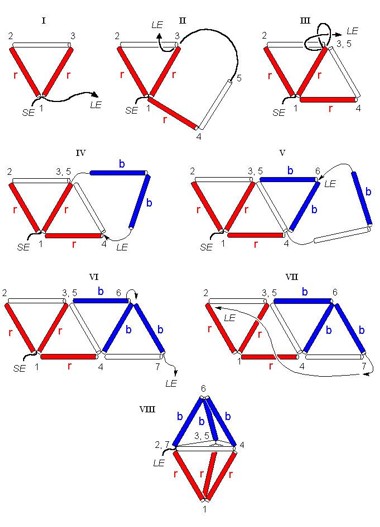
a). Building the trigonal bipyramid (Perez-Benitez,
2009).
In order to recognize the different parts of the trigonal bipyramid
(i.e. equatorial zone and apical vertices) is recommendable
to use colored drinking straws, so put attention in the order
in which the straws are joined (Figure
5):
Materials and tools:
9 drinking straws of 15 cm with colors: white (3), blue (3)
and red (3).
2 m of raffia.
1 scissors.
Instructions:
1. Insert the raffia into three drinking straws (red-white-red)
and make a knot for building the first triangle (Figure
5.I). Let one end to be short (SE) and the other one large
(LE).
2. In the LE insert two drinking straws (red-white) and pass the
raffia by the inner of the triangle at the point 3 (Figure
5.II).
3. Pull the raffia firmly and make a simple knot as it is illustrated
in the Figure 5.III
4. In the LE insert two drinking straws (blue-blue) and tie it
at the point 4 as it was mentioned above (Figure
5.IV).
5. In the LE insert two drinking straws (white-blue) and tie the
raffia at the point 6 (Figure 5.V).
6. Turn back the LE by the inner of the straw 6-7 and make a knot
at the point 7 (Figure 5.VI).
7. Finally join the point 7 with the point 2 and make a knot (Figures
5.VII-VIII).
8. Optionally, wooden sticks or straws cut along can be introduced
into the structure's straws to make it more consistent.
b). Building the reaction center.
Materials and tools:
1 plastic button (diameter = 3 cm).
4 standard metallic clips or 4 pieces of iron or copper wire (ca.
1.5 mm of diameter x 4 cm long).
1 insulated pliers.
1 alcohol burner.
Instructions:
Warning!: In this procedure you will use fire, so take the
required cautions.
1. Use the pliers to hold the wire and hot it for making three holes near to the perimeter of the button; distributed them in an equilateral triangular fashion (Figure 6a).
2. Insert one of the wires into a hole and tie it with the help of the pliers. Make a hook at the end of the wire. Repeat the same operation with the other two button's holes (Figure 6b).
3. This hook will serve to link one of the rubbers.
4. Take a piece of wire (ca. 4 cm long). Hot and insert it at the center of the button and make a hook at its ends (Figure 6c).
c). Building the substituents.
Materials and tools:
5 plastic bottle caps of different colors.
5 rubbers of number 18 (ca. 8 cm long in its resting position).
1 basic screwdriver.
1 insulated pliers.
1 alcohol burner.
Instructions:
1. Hot the point of the screwdriver and use it to make two
slots in all of the caps. The slots must be made near the top
center of the caps (Figure 6d).
2. Optionally, the caps can be labeled with numbers 1-3 and symbols
Lg and Nu for a kindly determination of the chirality sense.
d). Assembling the parts of the model.
1. Pass an end of a rubber around an equatorial vertex (Figure 6e). Turn around the other end, pass it by the inner of the rubber and pull it (Figure 6f).
2. Hang the free end of the rubber at the hook of the center atom. Repeat the same procedure with the other two equatorial vertices. Once the equatorial rubbers are connected to the central atom, use three pieces of raffia to tie permanently the caps (See Figures 4b and 4h).
3. The other two caps must not be tied in a permanent way because they will represent to the Nu and Lg groups; so they have to be removable. With this purpose pass the rubber by the inner of the cap's slots and make a single knot as it is mentioned in the step 1 (Figure 6g). Repeat the same procedure for the resting cap.
4. For a tetrahedral arrangement hang a rubber's free end (underside of the Figure 6g) to an apical apex of the central atom. Pull it by the extreme in which the cap is and hang it at the apical vertex of the bipyramid.
5. For a trigonal bipyramid arrangement connect the other cap as was mentioned above.
6. For a trigonal plane arrangement unplug the two apical caps.
5. Classical examples of reactions that occur under the SN2 and SN1 mechanisms.
a). The synthesis of n-butyl bromide (SN2
mechanism).
N-butyl bromide (1-bromobuthane) can be synthesized by
heating n-butyl alcohol (butan-1-ol) in the presence of
hydrobromic acid generated in-situ by the reaction of sodium
bromide with sulfuric acid (reaction 1). The experimental procedure
and the way of determining the kinetic of the reaction is described
in the literature (Pavia, 2005).
b). The synthesis or the hydrolysis of tert-butyl chloride
as examples of SN1 mechanism.
Either t-butyl alcohol (2-methylpropane) or tert-pentyl
alcohol (2-methylbuthan-2-ol) can be used for the synthesis of
the corresponding alkyl chlorides by an SN1
mechanism. The reaction occurs in a few minutes in the presence
of concentrated hydrochloric acid (reaction 2) and it can be carried
out by shaking the chemicals in a separatory funnel. The experimental
procedure and the way of determining the kinetic of the reaction
is described in the literature (Pavia,
2005).
The opposed reaction, it means, the hydrolysis of tert-butyl chloride to produce the tert-butyl alcohol (reaction 3) also proceeds by a SN1 mechanism. The procedure to determine the kinetic of the reaction can be verified on-line (Keusch, 2008).
6. Conclusion
The construction of this dynamic model have had good acceptance
by our students who build it in a workshop either in the classroom
or in the laboratory (ca. 1 - 1.5 h). In the classroom
we complement this activity with the calculation and the measurement
of the bond C*-Rx, both in the tetrahedral as in the trigonal
plane arrangements of our model.
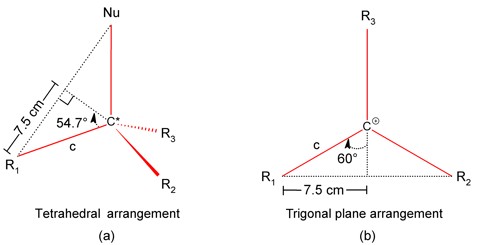
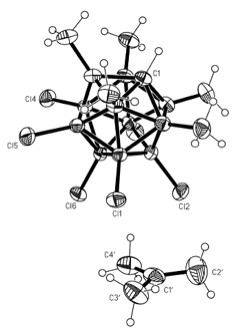
The calculation can be done by using the auxiliary constructions given in the Figures 7a and 7b, respectively. The use of the function sin 54.7° gives a value of 9.18 cm for the hypotenuse "c" at the tetrahedral arrangement (Figure 7a); and if it is used the function of sin 60° and the equivalence 9.18 cm = 1.54 Å for a C-C single bond (C*-Rx bond) it can be found the value of "c" = 8.66 cm = 1.45 Å, what is in a good agreement with the average value 1.442(4) Å found in the tert-Butyl cation: [tBu][CHB11Me5Cl6].CH2Cl2 (Figure 8; Kato, 2004).
The above mentioned means that our simple model is nicely coincident
with the carbocation experimental data. However it can be predicted
that the same thing will not occur for the SN2
mechanism, in which the C*-Rx bond in the transition state has
to be larger that the same bond in the initial state due to the
relaxation that is necessary for the entrance of the nucleophile.
In contrast, the model is very useful for illustrating the Walden's
inversion.
7. References
Anderson, M. M. (1987). "Two
working models for the SN2 mechanism". J. Chem. Educ.
64, 1023.
Berger, D. J. a). The SN1 reaction. On-line: <http://www.bluffton.edu/~bergerd/classes/CEM221/sn-e/SN1-1.html>; b). The SN2 reaction. On-line: <http://www.bluffton.edu/~bergerd/classes/CEM221/sn-e/SN2-1.html>. Last visited on July 21st, 2008.
Buist, P. H.; Raffler, A. A. (1991). "Dynamic molecular model". On-line: "Dynamic molecular model - Patent 5030103" <http://www.freepatentsonline.com/5030103.html>. Last visited on July 21st, 2008.
Chapter 4: SN1 mechanism. On-line: <http://library.tedankara.k12.tr/carey/ch4-3-1.html>. Last visited on July 21st, 2008.
Chapter 4: SN2 mechanism. On-line: <http://library.tedankara.k12.tr/carey/ch4-3-3.html>. Last visited on July 21st, 2008.
Fox, M. A.; Whitesell, J. K. (2004). "Organic chemistry". Sudbury, Mass: Jones and Bartlett. USA. Chapter 8.
Garrett, J. M.; Griffin, E. L. (1978). "Inexpensive model for illustrating stereochemistry of SN1 and SN2 reactions (old music stands never die-they just invert away) (CEC)". J. Chem. Educ. 55, 516.
Hamon, D. P. G. (1970). "A model to demonstrate the Walden inversion". J. Chem. Educ. 47, 398.
Hornback, J. (2005). "Organic chemistry". 2nd Ed. Thomson Brooks/Cole. USA. Chapter 8.
Kato, T.; Reed C. A. (2004). "Putting tert-Butyl Cation in a Bottle". Angew. Chem. Int. Ed. 43, 2907-2911.
Keusch, P. "Kinetics Hydrolysis of Tertiary Butyl Chloride - First Order Reaction". On-line: <http://www.chemie.uni-regensburg.de/Organische_Chemie/Didaktik/Keusch/cassy_tert.bucl-e.htm>. Last visited on July 21st, 2008.
Newman, M. S. J. (1975). "A molecular model for SN2 reactions" J. Chem. Educ. 52, 462.
Noller, C. R. (1947). "A simple apparatus to demonstrate Walden inversion". J. Chem. Educ. 24, 277.
Nyquist, H. LeRoy. (1965). "A simple model for the Sn2 mechanism". J. Chem. Educ. 42, 103A.
Pavia, D. L. (2005). "Introduction to organic laboratory techniques: a small scale approach". Brooks/Cole laboratory series for organic chemistry. Belmont, CA: Thomson Brooks/Cole, pp. 181-193.
Perez-Benitez, A.; Arroyo-Carmona, R. E.; Gonzalez-Vergara, E. (2009). "A simple system (named polyfacil) for building three-dimensional models of polyhedra starting from drinking straws and raffia". Chem. Educ. J. 12, Registration No. 12-1.
Sands, R. D.; Dressman, D. C.; Wyatt, S. R. (1995). "A model to show the SN2 inversion". J. Chem. Educ. 72, 428.
Sone, Y.; Sone, K. (1973). "SN1 and SN2 reactions. Paper marionette for demonstration". J. Chem. Educ. 50, 615.
Wade, L. G. Jr. (2004). "Quimica orgánica" 5ta. Edicion. Ed. Pearson Educacion, Madrid, p. 237.