Chemical Education Journal (CEJ),
Vol. 13, No. 2 /Registration No. 13-16 /Received November 25,
2009.
URL =
http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Usually, 2-fold improper rotation axis, S2, is considered as an inversion center, i, and vice versa. However, not only the geometrical representation of both symmetry elements is quite different but also an infinite number of S2 can be found in some molecules whereas there is only one inversion center in them.
To avoid confusion in the students, it is suggested that the teaching-learning of improper rotation axis only for n>2 should be recommended in undergraduate chemistry classes; otherwise, the restrictions of the equivalence S2 = i must be mentioned. A more successful discussion of this topic can be made with the help of two decorate cube models (belonging to C2h point group) whose templates are supplied in this article.
Resumen (pdf, in Spanish)
a) The construction of two decorated cubes to study C2h point group
b) The construction of a three-dimensional model of meso-tartaric acid
Symmetry, the property of an object to possess well balanced or well proportioned parts, is a fundamental concept in arts and sciences, since literature (e. g. left to right symmetry reading in palindromes; Scheme 1a) to symmetrical topologies in nature (e. g. 6-fold symmetry in snowflakes; Scheme 1b).
| "A man, a plan, a canal - Panama! Epitaph to Ferdinand de Lesseps (1805 - 1894), who was associated with the construction of the famous Panama's canal. |
 |
|
|
|
Scheme 1 Two examples of symmetrical entities: A palindrome (a) and a snowflake (b) (Taken from Libbrecht, 2009).
In chemistry, symmetry is of particular importance in the determination of many molecular properties, such as polarity, chirality, reactivity, absorption spectra, etc.
On the other hand, many authors of organic, inorganic or even group theory textbooks define the symmetry elements and symmetry operations as follows:
A symmetry operation is an atom-exchange operation performed on a molecule in such a way that, after the interchange, the shape and orientation of the molecule are not altered although the original position of some or all of the atoms may be occupied by the equivalent atoms. The realization of such type of processes is carried out by taking as a base a geometrical element (named as symmetry element): a point, a plane or a line. Thus, these concepts are intricately connected.
There are two classes of point symmetry operations: Proper and improper. The proper symmetry operations include the identity element, E (from the German word Einheit = unity) and the proper rotation axis, Cn, while the improper ones include the reflection plane, s, the inversion center, i, and the rotation-reflection axis (or improper rotation) operation, Sn (For a detailed explanation, see for example Miessler, 2004a). These operations are exemplified with (E)-1,2-dibromo-1,2-dichloroethene in Scheme 2.
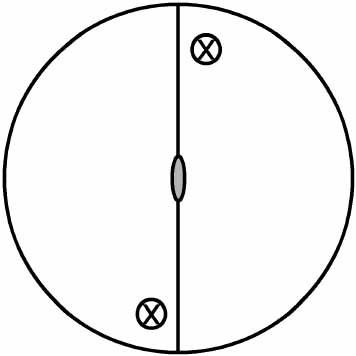
Scheme 2 Symmetry operations in (E)-1,2-dibromo-1,2-dichloroethene: Proper symmetry operations E and C2 (a counterclockwise rotation by 180°) are illustrated in the first row while the improper sh and i are in the second row. Note the equivalence between i (middle right) and S2 (bottom).
A rotation-reflection axis, Sn,
is by definition a combined operation composed of a proper rotation
axis, (Cn = 360°/n), followed
by a reflection on a perpendicular plain to the Cn:
Sn = Cn +
s![]() Cn
Cn
It is important to note that the existence of a Sn does not necessarily imply the existence
of the corresponding Cn and s as symmetry elements, which makes the
finding of a Sn difficult.
In particular, many authors consider that the operation S2 is equivalent to the application of i
as it is illustrated in the third row of Scheme
2:
S2 = C2 + s![]() C2
= i
C2
= i
However, in the search of the symmetry elements of the molecule
drawn in scheme 2, one of my inorganic undergraduate students
(first author in this paper) made me note in our class about the
"existence" of two additional S2
perpendicular to that mentioned above (Scheme
3).
Scheme 3 Location of two S2 contained in the molecular plane, which are orthogonal to that showed at the bottom of Scheme 2.
An exhaustive search led us an infinite number of new S2 which are located between S2 and S2'.
Bearing in mind this fact, the obligated question is: How many S2 should we take into account?
The answer is given by that mathematical property of point groups that says: "The number of proper operations, NOP, in an improper group is exactly equal to the number of improper operations, NIP ".
Thus, the two proper (E, C2) and two improper (sh and i) symmetry operations complete the set of operations that compose the point group C2h to which our molecule belongs (Scheme 4; Kettle, 2007; Vanovschi, 2009; Willock, 2009). Because the results of i and any of the S2 are really equivalent, the next question is: Which of all of the S2 must be taken into account?
Usually, the authors of many organic and inorganic chemistry and even group theory textbooks assign the axis perpendicular to the molecular plane as z-axis. Thus S2 is located perpendicular to the plane of paper as appeared in the stereographic projection for the C2h point group (Scheme 4b).
Finally, it is necessary to note that some authors have already perceived this problem. For example, Boeré mentions the redundant character of S1 (= s) and S2 (= i) and teaches Sn for n = 3, 4, etc. (Boereé, 2009) and Miessler comments that s and i are preferred over S1 and S2 (Miessler, 2004b).
|
 |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Scheme 4 Multiplication table (a), sterereographic projection (b) and character table (c) for the C2h point group (Atkins, 2006; web project, 2009).
Scheme 5 shows some examples of
chemical entities which belong to C2h
point group. You are invited to find in them, at list "three"
S2 which are collinear with the
x, y, z-axes, but if you prefer to realize an objective activity
then build the models suggested in the next section.
|
a) Hydrogen-bonded acetic acid dimers (Dreyer, 2005) |
|
b) (E)-1,2-diphenylethene (Baraldi, 1987) |
|
c) Ball-and-stick model and Schlegel diagram of Fullerene C2h-C48 (Fowler, 1995) |
|
d) (E)- Bis(ethylenethio)tetrathiafulvalene (BET-TTF) (Perez-Benitez, 1999) |
|
e) Ball-and-stick model and graphical representation of two symmetry elements (C2 and sh) of Si2H2: (Bogey, 1991; Chesnut, 2002; Sekiguchi, 2004) |
Scheme 5 Examples of chemical entities belonging to C2h point group.
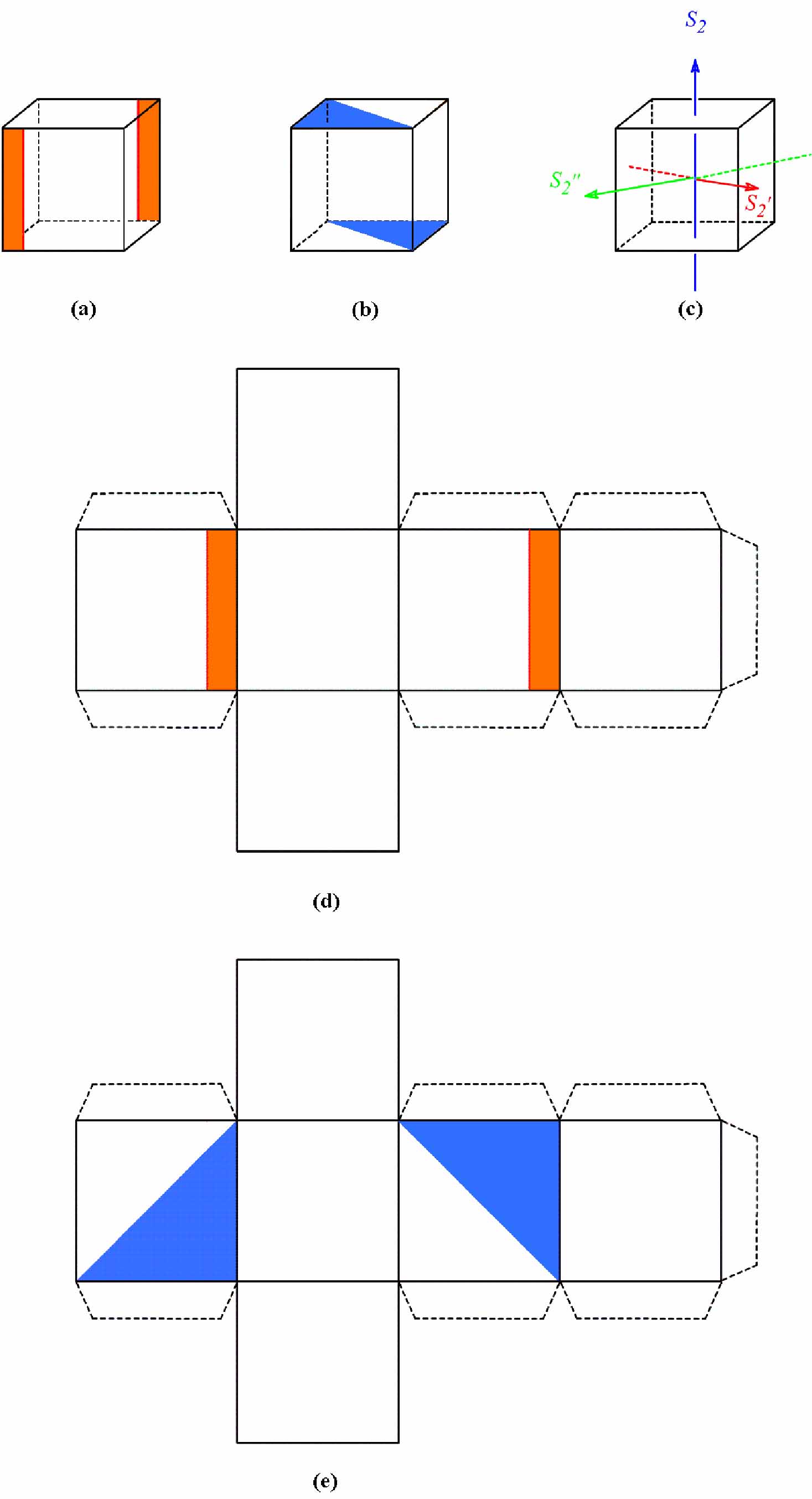
Scheme 6 Templates for building decorate cubes (a-b) in which at least "three" S2 can be located (c). Cut out the templates (d-e). Turn the flaps inwards and glue the models separately.
Consider the anti-periplanar conformer of meso-tartaric acid (Scheme 7c-d) and with the help of a flow diagram (Booth, 2004; Perez-Benitez, 2010a) determine its point symmetry. For a suitable search of more than one S2 in this molecule, the construction of a physical molecular model is widely recommended (Use for example, the paper model of meso-tartaric acid described by Perez-Benitez, 2010b).
Scheme 7 Meso-tartaric acid drawn in Newman (a, d), Fischer (b) and sawhorse (c) projections. The syn-periplanar conformer (a, b) posses a symmetry plane whereas the anti-periplanar conformer (c, d) has an inversion center.
Paradoxically to the general acceptance of S2
= i, there can be an infinite number of S2
in some molecules but only one inversion center, fact that is
supported by the definition of an improper group's order (the
number of proper operations is equal to the number of improper
operations). On the other hand, since the improper symmetry element
S1 is reduced to a plane of symmetry
(S1 = C1 + s![]() C1
= E + s = s), the application of C1
is worthless. Thus, the improper symmetry operations can be divided
in two subclasses: Subclass 1) It includes to s
and i, operations which are carried out in one step: The
reflection on a mirror plane and the inversion through the center
of symmetry, respectively; and subclass 2) It includes to those
symmetry operations which are carried out in two steps: Cn + s
C1
= E + s = s), the application of C1
is worthless. Thus, the improper symmetry operations can be divided
in two subclasses: Subclass 1) It includes to s
and i, operations which are carried out in one step: The
reflection on a mirror plane and the inversion through the center
of symmetry, respectively; and subclass 2) It includes to those
symmetry operations which are carried out in two steps: Cn + s![]() Cn = Sn
(where n
Cn = Sn
(where n![]() 3). Although this teaching-learning
approach is currently used by several authors, none of them had
provided a solid argument to support this classification.
3). Although this teaching-learning
approach is currently used by several authors, none of them had
provided a solid argument to support this classification.
Finally, if you carried out the analysis proposed in 5b (Scheme 7c-d) and/or a similar activity for the trans-diaminedichlorodinitroplatinum complex (Scheme 8; North, 1998) you will find that the existence of several S2 is not restricted to those molecules belonging to C2h point group but to all of the molecules possessing an inversion center.
Scheme 8 Bold wedge projection of trans-diaminedichlorodinitroplatinum complex