Chemical Education Journal (CEJ),
Vol. 13, No. 2/Registration No. 13-19 /Received March 2, 2010.
URL =
http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Formal Charge
Monoplayer Molecules
Multiplayer Molecules
Empirical Rules
Chemistry is an experimental science. Correlation of a range of experimental observation is possible with a number of qualitative concepts that are supported by appropriate theories. Structural formulae showing the arrangement of chemical bonds play a central role in such an approach and it is useful to establish the concepts involved.
The earliest description of the formation of a covalent bond is that of a shared electron pair. Lewis structures depict schematically how such pair are shared and give a topological picture of bonding in a molecule. Lewis concept [1] is the very direct interpretation of a bond in term of electrons, and even in modern discussion, it is quite often helpful to make use of Lewis's model.
Lewis structure is a preliminary step to write a most plausible molecular structure for a given molecular formula, before confirming through experimental means. Lewis theory of covalent bond formation is used to tackle this problem. The essence of this theory is to picturise covalent bond as (i) two-centre two electron bond and (ii) atoms with octet electronic structure.
The most plausible Lewis structure should be able to explain its actual shape and geometry when hybridization and valence shell electron pair repulsive model (VSEPR) concepts are applied. It should also explain bond parameters in a qualitative way from the resonance concept. Then it can be said that the method adopted in Lewis structures is acceptable. Although quite a few articles on Lewis structures have appeared in literature [2-9], a comprehensive method is yet cited. Writing Lewis structures becomes increasingly difficult as the number of atoms in a molecule or molecule ion increases. The topic dealing with Lewis theory is not vividly and elaborately presented in most of the general chemistry text-books and too much emphasis is often laid on the original concept of octet rule. Also modern Lewis concept of hyper and hypo valency is not emphasized. A comprehensive treatise for dealing with Lewis structure has not been dealt with in textbooks. As a result, many students of chemistry resort to a hit and trial method to write Lewis structure. Hence, the need for a student - friendly approach has arisen.
This paper attempts to overcome this difficulty of writing a Lewis structure firstly to select a central atom and then apply the given empirical rules. The modern theories of Lewis structure uses the concept of formal charge (FC).
Formal Charge is the residual charge on an atom in a Lewis structure. It may be zero, positive or negative. FC depends on number of valence electrons of an atom present in the Lewis structure.
FC = nVE - nLP - nLP
Where nLP = number of valence
electron; nLP = number of lone
pair electrons;
nBP = number of bonded pair electrons.
FC is helpful in choosing between more than one possible Lewis
structures. It is generally the case that the lowest energy structure
is the one with the smallest formal charge on the atoms (and which
usually lies between-1 and +1). For example, for Formaldehyde,
CH2O the following two possible structures
can be written as:
|
|
|
|
|
|
In both structures, each H atom has a FC of zero. In structure (a), the O atom has a FC of 6 - 2 - 1/2 (6) = + 1 and C atom has a FC of 4 - 2 -1/2 (6) = - 1
In structure (b), the FCs on O and C are 6 - 4 - 1/2 (4) =
0, and 4 - 0 -1/2 (8) = 0, respectively. Therefore, structure
(b) is most plausible.
If FCs are inevitable, in all of the plausible Lewis structures,
those with minimum FCs are to be considered. Generally negative
FC should reside on more electro-negative atom and positive FC
should reside on less electronegative atom. Sum of all FCs is
equal to zero for neutral molecule.
The charge of a molecular ion is equal to
the sum of FCs.
Second period element like C, N, O and F obey the octet rule
quite well. However, third period and subsequent elements show
deviations from it. For example PCl5, SF6, IF7 in which, the
central atom has 10, 12, 14 electrons respectively. These are
called hyper valent compounds.
The occurrence of hyper valence is widespread for the elements
of periods 3 to 6.
| 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 | |
| 2nd | Li | Be | B | C | N | O | F | Ne |
| 3rd | Na | Mg | Al | Si | P | S | Cl | Ar |
| 4th | K | Ca | Ga | Ge | As | Se | Br | Kr |
| 5th | Rb | Sr | In | Sn | Sb | Te | I | Xe |
| 6th | Cs | Ba | Tl | Pb | Bi | Po* | At* | Rn* |
Hyper valency is due to availability of low lying unfilled
"d" orbitals, which can accommodate the additional electrons.
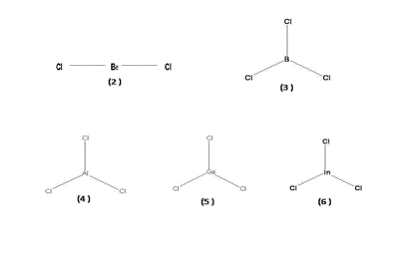
Cases of hypo valency central atom (<8 electrons) are also
known. For example BeX2, BX3,
AlX3, GaX3, InX3, (X = halogen) in which the central atom has
4, 6, 6, 6, 6, respectively. Hypo valency is the feature of Be,
B and 13 and 14 (Sn and Pb) group elements.
Molecules may be identified as mono- and multiplayer molecules. Monoplayer molecules are those in which all other atoms around the central atom are bonded. Multiplayer molecules are those in which more than one central atom is present. The central atom of monoplayer molecules can be identified based on electro negativity, size, higher oxidation state, presence of "d"-orbitals and period it belongs to [10, 11]. It is difficult to identify the central atom for complex (multiplayer) molecules. This task of identifying the central atom can be overcome from the following guidelines.
| Thiazylfluoride | NSF |
| NitrosylChloride | NOCl |
| Nitrylchloride | NO2Cl |
| Cyanate | (OCN)- |
| Isocyanate | (NCO)- |
| Thiocyanate | (SCN)- |
| Thiosulphate | (S2O32- = SSO32-) |
|
|
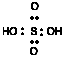 |
In selecting the central atom, the minimal FC rule is to be kept in mind mandatorily.
The problem in identifying central atom is easy if multiplayer molecule is written in terms of monolayer fragments.
(1) Split the molecule into equal halves; identify the central
atom in each fragment from the rules mentioned above.
| H2O2 | HO - OH | N2H4 | H2N - NH2 | |
| S2Cl2 | ClS - SCl | S2O42- | -O2S - SO2- | |
| S2O62- | -O3S - SO3- | Se2F6 | F3Se - SeF3 | |
| N2O4 | O2N - NO2 | P2O64- | 2-O3P - PO32- | |
| Cl2O4 | O2Cl - ClO2 | I2Cl6 | Cl3I - ICl3 | |
| H2S2O6 | HO3S - SO3H | C2H4 | H2C = CH2 | |
| C2H6 | H3C - CH3 | C2H2 | |
(2) If the molecule cannot be split into equal halves, it can be split into equal halves by placing an/all extra atom/s between two equal fragments.
| N2O5 | O2N - O - NO2 | P2O5 | O2P - O - PO2 | |
| I2O5 | O2I - O- IO2 | Cl2O7 | O3Cl -O-ClO3 | |
| P2O74- | 2-O3P - O - PO32- | Cr2O72- | -O3Cr - O - CrO3- | |
| H2S2O7 | HO3S - O - SO3H | S3O62- | -O3S - S - SO3- |
(3) If it is an acid and its basicity is known, O, H atoms can be written in the form of - OH group
| H3PO4 | (HO)3 PO | H3PO3 | (HO)2 H PO | |
| H3PO2 | (HO)2 PH | H2SO4 | (HO)2 SO2 | |
| H3BO3 | (HO)3 B | H4SiO4 | (HO)4 Si | |
| HClO4 | HO ClO3 | H2CO3 | (HO)2 CO | |
| H2S2O7 | HO-SO2 - O - SO2-OH |
(4) In peroxy and disulfide compound O - O and S - S single bonds are present
| Hydrogen peroxide | H2O2 | HO - OH |
| Hydrogen disulfide | H2S2 | HS - SH |
| Peroxysulphuric acid | H2SO5 | (HO) (HO-O) SO2 |
| Peroxydisulphuric acid | H2S2O8 | O - O (SO2-OH)2 |
|
HO- SO2 - O - O - SO2 -OH |
||
| Peroxydisulphuryl difluoride | S2O6F2 | FO2S - O - O - SO2F |
| Peroxyphosphoric acid | H3PO5 | (HO)2 PO (O-OH) |
| Peroxydiphosphoric acid | H4P2O8 | O - O (PO (OH)2)2 |
|
(HO)2 (O) P - O - O - P (O) (OH)2 |
||
| Bis(pentafluorosulphur) | S2F10 | F5S - SF5 |
(5) If the root nomenclature of the molecule is mentioned, its central atom can be easily identified based on its root structure.
| Aminosulphuric acid | NH2SO3H | (HO) SO2 (NH2) |
| Chlorine perchlorate | Cl2O4 | Cl ClO4 |
| Thiosulphate | S2O32- | S SO32- |
| Chlorosulphuric acid | HSO3Cl | (HO) SO2 (Cl) |
| Dithionate | S2O62- | -O3S - SO3- |
| Tetrathionate | S4O62- | -O3S - S2 -SO3- |
| Cyanamide | CNNH2 | NC - NH2 |
| Methylsulphuric acid | CH3SO3H | (CH3) SO2-OH |
Lewis structures for few case examples arrived at, using above guidelines and empirical rules are presented below.
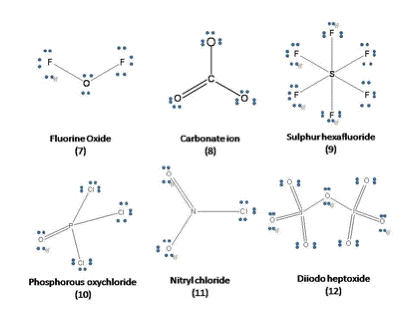
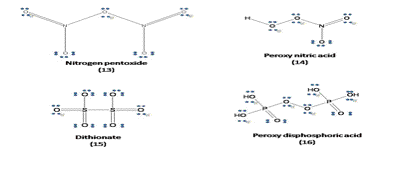
The objective of this article to help the student overcome the difficulty in writing the Lewis structure for octet, hyper and hypo valency compounds is hopefully achieved.
1. Bader, R. F. W., Atoms in molecules,
Oxford University Press, 1990.
2. Lever, A. B. P., J. Chem. Educ.,
1972, 49, 819-821.
3. Clark, T. J., J. Chem. Educ., 1984, 61, 100.
4. Zandler, M. E. and Talaty, E. R., J. Chem. Educ.,1984,
61,124 -127.
5. Carroll, J. A., J. Chem. Educ.,1986, 63, 28 -
30.
6. DeKock, R. L., J. Chem. Educ., 1987, 64, 934
- 941.
7. Sanadden, R. B., Educ. Chem., 1987, 24, 81 -
83.
8. Malerich, C. J., J. Chem. Educ., 1987, 64, 403.
9. Pardo, J. Q., J. Chem. Educ., 1989, 66, 456 -
458.
10. Ahmed, W. and Siraj, Omar, J.
Chem. Educ., 1992, 69, 791 - 792.
11. Ahmad, W. Y. and Zakaria, M. B.,
J. Chem. Educ., 2000, 77, 329 - 331.