Chemical Education Journal (CEJ),
Vol. 13, No. 2 /Registration No. 13-20 /Received December 4, 2009.
URL =
http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
This paper describes a new strategy for effectively implementing cooperative learning called "Rounding Cooperative Learning". This strategy was studied using teams in two groups; an experimental group and a control group. The analyses showed that the median student performance in the experimental group, on which the new strategy was applied, was 20 percentile points higher than that in the control group. The application of the Student t-test to the results of the experimental and control groups was 4.47 and 3.71, and the p-values were 0.001 and 0.003. These statistical results prove the efficiency of the strategy. The outcome of the strategy was extracted and concluded from questionnaires. Using this strategy, individual accountability, social and communication skills, thinking and learning styles and positive interdependence can be enhanced. This strategy can reduce poor interactions and help better the utilization of lab time. However, this strategy cannot be useful for long term projects.
Keywords: Rounding Cooperative Learning, Effective Teaching, General, Chemical Education Research
2.1. Lab Work
2.2. Groups' Design
2.3. Data Collection
In education, much training effort is spent on how teachers should interact with students and is devoted to arranging appropriate interactions between students and textbooks, curriculum and other materials. However, there is a need to move from these traditional passive styles into active styles in which students interact. One of these is called cooperative learning, which can be defined as the strategy of teaching and learning in which groups of students work together to achieve a common goal or to create a meaningful project as well as to improve their academic achievement. Research has shown the positive effects of cooperative learning at the elementary school level [1, 2] and secondary school level [3]. Studies have also examined the effects on performance of cooperative learning at the college level, in the general chemistry class [4-6] and chemistry laboratory [7-10]. For obvious reasons the cooperative learning group is made of more than one student, basically because there should be a high achiever in the group that can learn the lesson and then help others.
The problem with labs and projects organizing in teams is that there is less individual accountability [11-12]. In such situations, some team members do the bulk of the work, others contribute little and understand little or nothing about the project or the experiment but everyone gets the same grade [13]. Adjusting the grades for individual performance goes a long way toward correcting these injustices. In the case of lab teaching, one way to overcome this problem is to have pre-lab assignments, quizzes or final lab exams to contribute toward the final lab grade. However, the most important aspect in learning is still not achieved, i.e., each student should contribute personally and actively to the group final result as well as interact positively with his/her team mates. Inside the lab, the group of students work together during the whole semester as fixed lab mates.
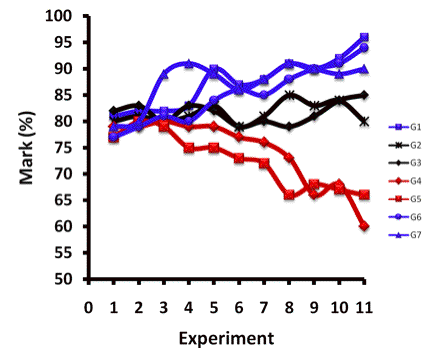
At King Fahd University of Petroleum and Minerals (KFUPM), to develop more positive attitudes about the first-year undergraduate chemistry course, and to encourage students to mutually help one another learn, we as authors of this manuscript applied the cooperative learning method in teaching the general chemistry laboratory. For the whole semester, students were assigned to work together in permanent groups to perform each experiment. The same standard was used to mark the report sheets. When the grades of students were analyzed at the end of the semester, it was found that the marks of some groups had gradually increased; in contrast, the marks of some other groups had continually dropped down. As Fig. 1 depicts, the marks of groups 4 and 5 gradually decreased. This most probably can be attributed to the weakness of the students of these groups. They stuck together during the whole semester with poor and negative interaction.
In response to this problem, a new strategy of learning and teaching was presented, applied and investigated. Trying to solve such cases, we suggested a method that can be called "Rounding Cooperative Learning". In this method, instead of fixing students in the same group during the whole semester, each student had to work with a different partner each experiment since each experiment could be performed on the same week. This paper describes the implementation of this strategy during two consecutive semesters.

Fig. 1 Representation of student- groups' marks before application of the strategy with G: group number
The lab work contains experiments respectively deal with basic operations such as how to find the density of solid and liquid samples, determination of total dissolved salts in water, identification of cations and anions, percentage of water in hydrated salt, limiting reactant, melting point of liquid, melting point of solid, calorimetry, synthesis of an alum, and volumetric analysis.
The study was run for two consecutive semesters. Each semester one laboratory section served as the control group and the other as the experimental group. Students did not know which section was the experimental group when they registered. Both sections were taught using the same laboratory experiments but the experimental group was given instructions for each student to work with a different partner each experiment, i.e. rotating or rounding. Student number one, for example, worked with 11 different students during the whole semester. Each student had to submit an individual lab report.
Data collection was designed to provide information about academic performance and students' attitudes. The data were collected from both control and experimental sections of the laboratory. In addition, questionnaires were distributed to students in the other sections where only cooperative learning was applied. EXCE and MINITAB softwares were used for analyses.
The evaluation of the rounding cooperative learning strategy consisted of two parts: the statistical comparison analysis of the laboratory grades of the experimental and control sections during two semesters, and the students' responses to the questionnaires.
An independent t-test was employed to determine whether there was a statistically significant mean difference between the control and experimental sections with respect to the laboratory grades. The data indicated that there was a significant difference between the experimental groups and the control groups (t = 4.47, p = 0.001 in the first semester, t = 3.71, p = 0.003 in the second semester of the conducted study) (see Table 1). Students' means in the experimental sections were significantly higher than those in the control sections. The effect size was calculated according to the following equation [14]:
![]()
The effect size shows that rounding cooperative learning can significantly enhance student efficiency and understanding in the laboratory. An effect size of 0.54 means that median students' performance was significantly improved from the 50th percentile in the sections taught by cooperative learning without rounding to approximately the 70th percentile with rounding cooperative learning.
Table 1 Comparative Statistical Analysis
|
|
|
|
Effect Size |
|
|
| Semester 1 | Rounding Cooperative Learning |
|
|
|
|
| Cooperative Learning |
|
||||
| Semester 2 | Rounding Cooperative Learning |
|
|
|
|
| Cooperative Learning |
|
* t-test critical value is 2.1788 ( at P = 0.05)
Table 2 Students' Responses to the Questionnaires
| Working with different partner each experiment: |
|
|||||
|
|
|
|
|
|
||
| 1 | increases positive interaction with different classmates which enhances the mutual help in understanding the contents of the experiments |
|
|
|
|
|
| 2 | improves understanding of experimental skills (how to perform the steps of the experiments, titration, evaporation, measurements, ...) |
|
|
|
|
|
| 3 | increases social interaction which enhances the chance to discuss different subjects outside the class and get help not only in this course but also others |
|
|
|
|
|
| 4 | reduces the chance to discuss personal issues |
|
|
|
|
|
| 5 | improves the teamwork skills |
|
|
|
|
|
| 6 | increases positive interdependence |
|
|
|
|
|
* (SA): Strongly Agree, (A): Agree, (N): Neutral, (D): Disagree;
(SD): Strongly Disagree
** Short description was given before running this questionnaire
to familiarize students with its terminology
At the end of the course, students were given questionnaires to verify that they agreed with rounding cooperative learning strategy (Table 2). Most of the students who practiced rounding cooperative learning agreed with and supported the rounding strategy. This feedback showed that students were comfortable while working with a different partner each experiment and that the rounding strategy was beneficial for them. Also about half of the students who practiced cooperative learning with fixed labmate preferred having rounding strategy. Therefore, it can be inferred that general chemistry students perceived that understanding and learning can be improved when they perform each experiment with different partners.
From the statistical analysis results and the positive response to questionnaires, the outcome of rounding cooperative learning can be extracted as shown in Figure 2.
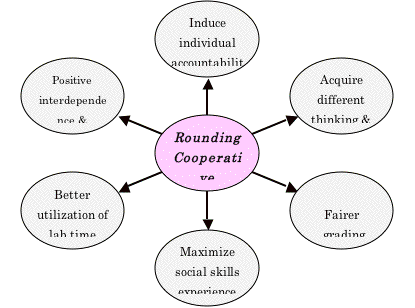
Inducing individual accountability: With normal cooperative learning, group members can easily shift their focus from learning to grade outcome, becoming concerned with grades, rather than with learning. On the other hand, when a student works with a new partner, he has at least two factors motivating him. The first factor is to show and prove his personality and skills to others. The second factor is to not rely on his partner to do every thing since he is not aware of the quality and level of his partner's work. Furthermore, in rounding cooperative learning, there are fewer poor interactions because both students shift to a new partner each lab and hence have a better chance for positive interactions.
Acquiring different thinking and learning styles: Students learn and think in different ways. There are different theories on learning styles which encourage lecturers to use different teaching methodologies to reach the maximum number of students. Some students analyze the results intuitively based on figures and drawings; they look at the whole picture. Others analyze their results logically based on numbers and facts, and they look at details. Dealing with different partners can be very useful in gaining new thinking and learning styles.
Fair grading: With normal cooperative learning many of the hard workers do most of the work and the lazy students do less and still receive a similar grade. However, this is not the case in rounding cooperative learning because the student has to work with a new partner each lab, and he interacts and does the job as there is no previous experience with dependence on this partner.
Maximizing experience in social skills: Sometimes students choose their lab mate because they are friends, are living in the same place, are from the same country or are doing the same major. If the student is assigned to work with a different partner each lab, he can win more friendships and discover many good qualities in his colleagues. Also he can interact and communicate with different personalities, which is a very important aspect of cooperative learning.
Activating positive interdependence & interaction: Each student relies on his partner to some extent and at the same time he does his own part in the best way he can to get the job done successfully. Neither partner depend on the other exclusively since they have no previous collaboration.
Better utilization of the lab time to finish the work: Both partners are serious and probably do not have common matters to discuss they work together the first time except a few questions to introduce themselves to each other. The rest of the time will be spent doing the experiment and analyzing the results.
The new strategy called rounding cooperative learning has the advantage of inducing individual accountability by varying the lab partner each experiment which reduces poor interactions and creates personal motivation for the student to positively cooperate with his lab mates. Also it can be effective in developing social and communication skills, thinking and learning styles, positive interdependence and better utilization of lab time. This strategy can help prevent a weak student from sticking with the same partner, and so reduce poor interactions for both. It can develop strong interaction between students and eliminate or reduce the chance to discuss personal issues. On the other hand, this strategy cannot be useful for long-term work, such as long experiments that require more than one set since partners are going to be change from one set or experiment to another. Also, this strategy cannot be useful for long-term work such as term projects.
The effectiveness of the rounding cooperative learning strategy is recommended to be studied in other subjects. More investigation is also required to be done in different chemical subjects and for different chemistry level. We recommend general chemistry laboratory teachers and instructors to utilize the strategy.
The authors are grateful to the reviewers for their helpful comments and guidance. Any opinions or suggestions can kindly be sent to the author via email and would be highly appreciated.