1 Escola Vera Cruz; Rua Baumann, 73, Sao Paulo - SP, 05318-000 Brazil.
2 Colegio Santa Cruz; Av. Arruda Botelho, 255, Sao Paulo - SP, 05466-000 Brazil.
3 Instituto de Quimica, Universidade de Sao Paulo, 26077 Sao Paulo - SP, Brazil.
e-mail: carmen
Chemical Education Journal (CEJ),
Vol. 13, No. 2 /Registration No. 13-22 /Received October 3, 2009.
URL =
http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Ana Luiza P. Nery1, Rodrigo M. Liegel2 and Carmen
Fernandez3
[*]
1
Escola Vera Cruz; Rua Baumann,
73, Sao Paulo - SP, 05318-000 Brazil.
2 Colegio Santa Cruz; Av. Arruda
Botelho,
255, Sao Paulo - SP, 05466-000 Brazil.
3 Instituto de Quimica,
Universidade de Sao Paulo, 26077 Sao Paulo - SP, Brazil.
e-mail: carmen![]() iq.usp.br
iq.usp.br
Keywords: curriculum; demonstrations; excited states; luminescence; photochemistry; UV-Vis Spectroscopy
Contents
Abstract
1. Introduction
2. Materials and Equipment
3. Description of the Experiments
a) Chlorophyll fluorescence
b) Brown eggshell fluorescence
c) Riboflavin (B2 vitamin) fluorescence
d) Tonic water fluorescence
e) Optical Whiteners
f) Chemiluminescence: Light sticks emission
g) Producing a light stick
4. Final Remarks
5. Safety
6. Acknowledgements
7. References
Abstract
The phenomenon of luminescence generated either by a chemical process (chemiluminescence) or by an external source of energy (fluorescence and phosphorescence) is an important analytical tool. Many biological substances emit characteristic fluorescence. Chlorophyll from leaves emits red fluorescence; protoporphyrin IX, found in the brown chicken eggshell and riboflavin (B2 vitamin) also exhibit characteristic fluorescence emission. Substances present in detergents (optical whiteners) are responsible for the fluorescence observed in white clothes from people dancing under black light. Light sticks, objects used by kids, divers and campers owe their chemical ground to the chemiluminescence. Although the relevance of these phenomena, most chemistry curricula omit discussion about their photophysical principles, even though include some treatment of light absorption and spectroscopy. In general, the photophysical principles are left to graduate studies. We consider that, due to the visual appeal of these phenomena, it is possible to discuss basic photophysical principles in undergraduate level and, thus, we are suggesting simple demonstration experiments in this paper.
The phenomenon of luminescence is frequently omitted from most undergraduate chemistry classes, even though chemistry curriculum includes some treatment of light absorption and spectroscopy. On the other hand, due to its strong visual appeal, we consider that luminescent phenomenon could be used as a good strategy to teach basic photophysical principles even at undergraduate level. Moreover, luminescence is an important analytical tool, in particular for biomedical applications since luminescent probes can be used as sensors for chemical environment (Leiner, 1991, Lakowicz, 2006). This paper describes some simple demonstration experiments involving the phenomenon of luminescence generated either by a chemical process (chemiluminescence) or by an external source of energy (fluorescence and phosphorescence) in order to promote a discussion about photophysical principles in classroom.
Light that is incident on a colored sample is partly absorbed by it. The first step of a chemical or a physical process induced by light involves absorption of a quantum of light by a molecule, producing an electronically excited state. In this initial step the molecule absorbs a discrete amount of light, which is enough to promote an electron to a higher energy level; the photon must contain a quantity of energy that exactly matches the energy of the electronic transition. The molecule is said to go from the ground to an excited state. Once in the excited state, the molecule has several available pathways to release the absorbed energy. The result of the absorption may appear as heat or light. Following light absorption, different processes may occur: (i) nonradiative decay: the process of vibrational relaxation, in which the excess energy is transferred to the surroundings as thermal motion of the environment (heat); (ii) fluorescence or phosphorescence in which a photon of the same or lower energy is emitted while the molecule returns to the ground state; (iii) chemical change, when energy results in changes in bonding structures, or a combination of these. The total energy change of the processes is always exactly equal to the energy of the photon that was absorbed.
The photophysical processes occurring from absorption to emission are often shown in a so-called Jablonski diagram, which is a simplified representation of the relative positions of electronic and vibrational energy levels of a molecule (Figure 1). Of course all possible energy routes cannot be encompassed in a single figure, and different forms of the diagram can be found in different contexts.
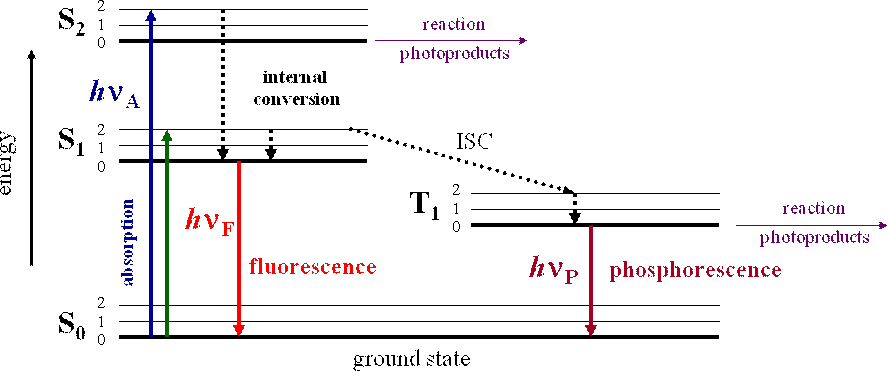
Figure 1. Simplified Jablonski diagram. The sequence of events leading to fluorescence and phosphorescence are shown. S0 is the ground state, S1 and S2 are excited singlet states; T1 is an excited triplet state. 0, 1, 2 represent vibrational levels. Straight lines represent transitions involving photons, dotted lines represent vibrational or thermal transitions.
Luminescence is defined as light emission in the visible range (400 to 700 nm) of the electromagnetic spectrum as a result of an electronic transition. Light-induced luminescence can be of two types: fluorescence or phosphorescence. Radiative transitions involve light emissions. Radiation is called fluorescence if spin multiplicities both in ground and excited state are the same.
Absorption of a photon (hnA in figure 1) populates the vibrational levels of S1 (or S2). This process is very fast and happens within 10-15 s. In the next 10-12 s the molecule relaxes to the lowest vibrational level of S1, a process called internal conversion. Since emission typically occurs after 10-9 s the molecule is fully relaxed at the time of emission, hence, as a rule, emission occurs from the lowest vibrational level of S1 (KashaLs rule) and the fluorescence spectrum is generally independent of the excitation wavelength. As a consequence, light emission accompanying decay of excited molecules is always at longer wavelength (lower energy) than that of the absorbed light. After emission (hnF in Figure 1) the molecule returns to the ground state, possibly after vibrational relaxation. This completes the simplest case of fluorescence: excitation, internal conversion, emission and relaxation. On the other hand, phosphorescence involves excited and ground states with different spin multiplicities. In this case, after excitation to a vibrational level of S2, for example, an intersystem crossing (ISC) to an electronic triplet excited state occurs. As a result, in contrast to fluorescence, in the phosphorescence phenomena light is emitted over a longer period of time since the excited molecule undergoes a transition to a different excited state; a metastable state. Metastable means that the electronic state is not completely stable, but it is also not particularly unstable either. Thus, not all electrons in the metastable state will return to the ground state at once. The radiative decay (hnP in Figure 1) from the triplet state is a slow process:the return to the singlet ground state is spin-forbidden. As the electrons of a phosphorescent molecule return to the ground state the molecule continues to emit light. Perhaps an easy way to identify fluorescence or phosphorescence is if you can see the emission after the illuminating source is removed, it is phosphorescence. If the emission stops when the illuminating source is removed, it is fluorescence. Phosphorescent objects should be familiar to almost everybody: stick-on stars, frisbees, cereal box goodies, watch faces, posters and so on.
Many ordinary materials such as green vegetables, tonic water, detergents, and brown eggshells, contain substances that may emit fluorescence under determined conditions. This paper describes experiments for teaching photochemistry in an undergraduate chemistry class, with the aim to discuss some photophysical processes, relating the observed properties of fluorescent compounds to energy levels diagrams and to absorption and emission spectra. Another important fact to be pointed out refers to the use chemical energy, besides electromagnetic energy, as excitation source.
Black light source: UV-A light (315 - 400 nm)
Mortar and pestle
Light sticks (different colors)
Ethyl acetate
Hydrochloric acid 0.1 mol L-1
3-5 spinach leaves
Brown eggs
Multivitamin tablet
Tonic water
Detergent
3. Description of the Experiments
For the following experiments all background light must be turned off and the room must be as dark as possible. The UV source must be kept in a box with a frontal slit to avoid students to look directly to the light, which may cause retina damages. It is important to compare the observed colors under visible and UV excitation, mainly in the case of chlorophyll, since its solution is green under visible light and emits in the red range of the visible spectra under UV excitation.
Chlorophyll, a natural pigment found in green plants is an example of a fluorescent compound. It is the most important light absorbing pigment in thylakoid membranes in the chloroplasts (Nelson and Cox, 2000). Chloroplasts of higher plants always contain chlorophyll a and b. The structure of the chlorophyll molecules consists of several conjugated nitrogen-containing rings surrounding a magnesium ion by coordinate covalent bonds (Figure 2). This structure resembles the protophorphyrin of the heme group of hemoglobin; one striking difference between chlorophyll and the heme group of hemoglobin (besides other structural differences) is that in chlorophyll a Mg2+ ion occupies the central position, instead of the Fe2+ ion that is present in hemoglobin.
|
chlorophyll a
|
chlorophyll b
|
. Structural formulas of chlorophyll a and chlorophyll b.
Although both forms of chlorophyll are green, their absorption spectra are sufficiently different to allow them to complement each otherfs range of light absorption in the visible region. The green color is due to absorbance range, which maxima are at 412 nm (blue region) and 666 nm (red region) for chlorophyll a (Figure 3).
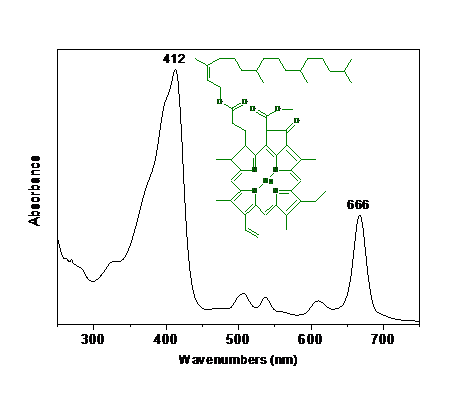
Figure 3 . Absorption spectrum of a chlorophyll a solution.
Comparing the color of chlorophyll solution under visible light and UV irradiation is an excellent opportunity to discuss light absorption and emission. Blue and red light absorption by chlorophyll (see Figure 3) results in a green solution (solution reflects green color). In the absence of visible light, under enough energy to promote an electronic transition red fluorescence is observed. Chlorophyll fluorescence occurs in the red region of visible light, between 675 and 685 nm. Fluorescence is not observed when the solution is concentrated, since the red emission is reabsorbed by the solution. The drastic change of color in the case of chlorophyll is an experiment that has a beautiful visual appeal, motivating students to pay attention to phenomena related to light absorption and emission.
Why does not chlorophyll fluoresce under visible light? As a matter of fact, it does. However, the higher intensity of the emitted green light does not allow the red fluorescence to be observed. If the solution were irradiated in a dark room with blue light, fluorescence emission would be observed.
Procedure: Grind fresh spinach leaves with a mortar and a pestle in about 50 mL of ethyl acetate. Transfer the green solution to a beaker. Irradiate it with the black light. If no fluorescence is observed, dilute the solution with more ethyl acetate and irradiate again. If the solution is too concentrated, no fluorescence can be observed, since absorption and emission spectra overlap and fluorescence reabsorption may occur.
b) Brown eggshell fluorescence
Protoporphyrin IX, the intermediate in the biosynthesis of heme group of hemoglobin, shows a very stark fluorescence under UV light excitation. In contrast to molecules with the heme group, in protophorphyrin IX structure there is no central metallic atom (Figure 4).
|
Protoporphyrin IX
|
Heme group
|
Figure 4 . Structural formulas of protoporphyrin IX and heme group.
Protophorphyrin IX is found in some bones and mollusk shells. It is responsible for the brown color of chicken eggshells. If an eggshell were irradiated with black light, a very weak fluorescence would be observed. However, after treatment of the eggshell with hydrochloric acid in ethyl acetate, one can immediately observe the formation of bubbles on the surface of the egg. The bubbles are carbon dioxide resulting from the reaction of the CaCO3 in the eggshell with hydrochloric acid:
CaCO3(s) + 2 HCl (aq) --> CaCl2 (aq) + CO2(g) + H2O (l)
As the eggshell reacts, protoporphyrin IX is liberated to the solution, and under UV light one can observe a peculiar spectacle of the gradual increase of the fluorescence emission from the solution. The previous very week fluorescence of the intact eggshell disseminates through the solution as an intense purplish emission (Brandl, 1998).
Procedure: Wash a brown eggshell and irradiate it with black light. Put the eggshell in a beaker, add about 50 mL of ethyl acetate and irradiate again. Add some drops of HCl 0.1 mol L-1 to the solution, irradiate again and observe fluorescence during gas liberation.
c) Riboflavin (B2 vitamin) fluorescence
Riboflavin (Figure 5), or vitamin B2, is a fluorescent compound found in many foods, such as milk and eggs and also on multivitamin tablets. Under UV-A excitation, its fluorescence is observed by naked eyes as an intense green-yellow emission (Chatellier and White, 1988).

Figure 5 . Structural formula of riboflavin.
Procedure: Grind a multivitamin tablet with a mortar and a pestle. Transfer the solid to a beaker and add about 50 mL of water. Irradiate it with black light.
May be one of the most known fluorescent ordinary products is tonic water, whose active ingredient is quininium cation (Figure 6).
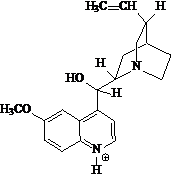
Figure 6 . Structural formula of quininium cation.
Quinine is added to the beverage as the hydrochloric or sulfuric acid derivative, giving its bitter flavor. Quinine is found in the root, bark and branches of cinchonas trees, native to South America and has been used as a treatment for malaria for hundreds of years. It does not cure the disease, but does help to suppress parasitic activity and thus reduce life-threatening fever ( Krettli et al., 2001). It is a strongly fluorescent compound in dilute acid solution emitting at ca. 450 nm and is used as an important fluorescence standard in chemistry (Pandey et al., 1999). Quinine solutions are colorless, which indicates that it has no meaningful absorption in the visible range and that excitation must necessary occur at UV region of the electromagnetic spectrum, within its absorption bands at 250 and 350 nm.
When NaCl is added to the solution, the fluorescence emission is reduced. This phenomenon, called fluorescence quenching, can be explained based on the Jablonski diagram. A quinine molecule in its excited electronic state can be deactivated by a collision with a chloride ion before it has a chance to return to its ground state by the emission of a photon. The chloride ion acts as a fluorescence quencher, reducing the fluorescence quantum yields. The fluorescence quenching of quinine can be related to the halide concentration, and can be used for the sensing of chloride ions for analytical and clinical purposes (Martin and Narayanaswamy, 1997).
Procedure: Pour tonic water into two jars and turn on black light. Add some NaCl to one of the jars and compare both samples. Discuss the observed phenomena: fluorescence and quenching on basis on the Jablonski diagram.
Another simple fluorescent experiment to be demonstrated is shining black light onto white clothes. The observed fluorescence is due to the presence of optical whiteners in detergents; a class of organic compounds that have very specific fluorescent properties. They have excitation maxima in the near UV (generally 340 - 400 nm) and emit in the 430 - 460 nm wavelength range, generally peaking around 440 - 450 nm.
Procedure: Put some detergent crystals under the black light and observe the fluorescence. Add the detergent into water and irradiate again.
f) Chemiluminescence: Light sticks emission
Many chemical reactions produce both light and heat. In these cases, the energy source that causes electrons to move from the ground to the excited state comes from the chemical reaction. A burning candle is such a reaction. When a candle is lit, its flame both glows and becomes hot. It is much less common for a chemical reaction to produce light without heat. Such reactions, where energy is given off as visible light, are called chemiluminescent reactions. Chemiluminescence is defined as the production of light energy from a chemical reaction without the use of heat, for this reason it is also known as "cold light". When light emission derives from living organisms the phenomena is called bioluminescence. Chemiluminescence is the chemical ground of light sticks, these objects that, besides wonder kids, are used by trick-or-treaters, divers, campers, and for decoration and fun. A CYALUMER (trademark of American Cyanamid Company) light stick is a plastic tube divided into two compartments. Although there is more than one recipe for a light stick, a common commercial one uses a solution of hydrogen peroxide kept in one of the compartments and a solution of a phenyl oxalate ester, for example bis(2,4-dinitrophenyl)oxalate (DNPO) or bis-(2,4,6-trichlorophenyl)oxalate (TCPO), together with a fluorescent dye (Fl) (Shakhashiri et al., 1981).
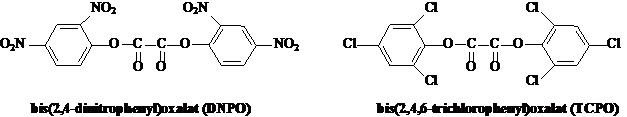
When a light stick is bent, the glass vial breaks, and the chemicals that are inside the glass mix with those in the plastic tube, allowing the chemical reaction to take place. The basic premise is that the reaction between the two chemicals releases enough energy to excite the electrons in the fluorescent dye (Fl). Scheme 1 shows chemiluminescent decomposition of TCPO (Mohan and Turro, 974):
Scheme 1 :
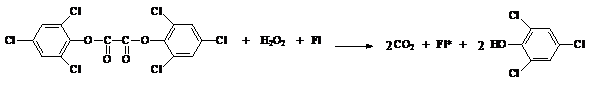
The emission of the fluorescent dye (Fl) is what determines the observed color as product of the reaction (Table 1).
Table 1: Fluorescence emission of selected dyes.
|
dye |
color |
|
9,10-diphenylanthracene |
blue |
|
9,10-bis(phenylethynilanthracene) |
green |
|
Rubrene |
yellow |
|
Rhodamine 6G |
orange |
|
Rhodamine B |
red |
Mechanistic studies indicate the occurrence of a Chemically Initiated Electron Exchange Luminescence (CIEEL) mechanism (Schuster, 1979), with the formation of a 1,2-dioxetanedione as intermediate, which transfers energy to the dye molecule. The dye molecule acts as a catalyst of the process. Light emission is observed when the excited molecule of the dye (Fl*) returns to the ground state (Stevani and Baader, 2000). This mechanism suggests that if the dye molecule (Fl) can be excited by some other energy source (UV light for example), the same light emission should result, even after reagents were totally given up. Salter et al. (1999) have observed that light sticks of different colors show very similar chemiluminescence and laser-induced fluorescence spectra.
Procedure: Irradiate different color light stick. Observe and discuss the observed emission. Break some of them. Discuss different kinds of excitation source: light and chemical reaction. Irradiate a light stick whose chemicals have already been given up.
Light stick reaction may be produced in class. In this case it is possible to produce other colors by using different dyes, for example chlorophyll solution, allowing comparison of two different processes: photophysical (fluorescence) and chemiluminescence. In a beaker containing about 100 mL of ethyl acetate, add 10 mL of 2.7 mol L-1 H2O2 aqueous solution and about 50 mg of a fluorescent dye. Add ca. 5 mg of TCPO or DNPO, turn off the lights and observe.
Basic notions of quantum chemistry, quantization of molecular energy levels and spectroscopy may help students to understand some everyday phenomena. The discussion of experiments like those suggested in this paper may be useful for the process of giving meaning to the surrounding world through chemical knowledge. The examples here discussed are a very effective pedagogical tool for the demonstration of two chemical phenomena involving light: fluorescence and chemiluminescence. Such phenomena have been widely used as analytical tools, mainly in biomedical analysis, due to their high sensibility.
Ultraviolet light is potentially damaging to exposed skin and especially eyes. Do not stare at the black-light source. Ethyl acetate is flammable. Hydrochloric acid is corrosive, can cause severe burns to the skin in its concentrated form, and should be handled with care. There are no significant hazards associated the fluorescent compounds.
The authors acknowledge financial support from FAPESP, CAPES, CNPq, Dow Chemical Company and Pro-Reitoria de Pesquisa (Universidade de Sao Paulo).
Brandl , H. (1998). Trickkiste Chemie, Munchen: Bayerischer Schulbuch Verlag.
Chatellier , D. S.; White, H. B. (1988). " What color is egg white? A biochemical demonstration of the formation of a vitamin-protein complex using fluorescence quenching". J. Chem. Edu . 65, 814-815.
Krettli , A. U.; Andrade-Neto, V. F.; Brandao, M. G. L.; Ferrari, W. M. S. (2001). Mem. Inst. Oswaldo Cruz 96 , 1033-1042.
Lakowicz , J. R. (2006). Principles of Fluorescence Spectroscopy, Berlin: Springer, Third Edition.
Leiner , M. J. P. (1991). "Luminescence chemical sensors for biomedical applications: scope and limitations". Anal. Chim. Acta 255, 209-222.
Martin , A.; Narayanaswamy, R. (1997). " Studies on quenching of fluorescence of reagents in aqueous solution leading to an optical chloride-ion sensor". Sensor Actuat. B 39, 330-333.
Mohan , A. G.; Turro, N. J. (1974). "A facile and effective chemiluminescence demonstration experiment". J. Chem. Edu. 51,528-529.
Nelson, N. L.; Cox, M. M. (2000). Lehninger Principles of Biochemistry.New York: Worth Publisher.
Pandey , S.; Borders, T. L.; Hernandez, C. E.; Roy, L. E.; Reddy, G. D.; Martinez, G. L.; Jackson, A.; Brown, G.; Acree, W. E., Jr. (1999). " Comparison of Analytical Methods: Direct Emission versus First-Derivative Fluorometric Methods for Quinine Determination in Tonic Waters." J. Chem. Edu . 76, 85-87.
Salter , C.; Range, K.; Salter, G. (1999). " Laser-Induced Fluorescence of Lightsticks". J. Chem. Edu . 76, 84-85.
Schuster , G. B. (1979). "Chemiluminescence of organic peroxides. Conversion of ground-state reactants to excited-state products by the chemically initiated electron-exchange luminescence mechanism". Acc. Chem. Res. 12, 366-373.
Shakhashiri , B. Z.; Williams, L. G.; Dirren, G. E.; Francis, A. (1981). ""Cool-Light" Chemiluminescence". J. Chem. Edu. 58, 70-72.
Stevani , C. V.; Silva, S. M.; Baader, W. J. (2000). "Studies on the Mechanism of the Excitation Step in Peroxyoxalate Chemiluminescence". Eur. J. Org. Chem. 24, 4037-4046.