Chemical Education Journal (CEJ),
Vol. 14, Issue 1 /Registration No. 14-4 /Received June 21, 2010.
URL =
http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
Scientific knowledge is
complex and dynamic in nature. To make the science teaching and
learning processes more meaningful, concepts are arranged according
to their difficulty for teaching at various grade levels and teachers
often try to make the complex explanations of the concepts very
simple for the students to help their understanding. The learning
of oversimplified concepts exhibit two main problems: (a) the
concepts lose their accuracy and the students learn misconceptions
(students now trust heavily on the accuracy of the taught and
learned information), and, (b) the information is not sustainable
and needs to be re-taught soon after. When teachers tell students
that what they have learned previously is no longer correct and
they have to learn the revised form of the concept again, students
often get frustrated and complain why they were taught the wrong
information. In this paper the author will argue a case about
how to teach (a) the definition of an element, (b) the electronic
configuration of the elements, and (c) the effect of mixing two
miscible liquids on their total volume for sustainable learning
to avoid the frustrations of students, as well as to save time
by avoiding the teaching, un-teaching and re-teaching cycle of
the same information.
Keywords: Chemistry, Element, Electronic structure of atom, sustainable learning, Miscibility of Fluids, Volume, High school chemistry, Brunei
Example 1: Definition of an Element
Science subjects in school have a reputation for being among the most difficult to learn (Fensham, 2002). For example, a number of studies show that many students at all levels struggle with chemistry (Coll & Treagust, 2001; Coll & Treagust, 2002; Dhindsa, 2000; Dhindsa, 2002; Nakhleh, 1992). The inadequacy in students' chemistry content knowledge has been a major concern to educators (Dhindsa, 2002). A number of studies have been conducted to discover why many students at all levels struggle with chemistry (Coll & Treagust, 2001; Coll & Treagust, 2002; Dhindsa, 2002; Dhindsa & Treagust, 2009; Nakhleh, 1992). A large quantity of scientific information is taught in a grade level; its abstractness, the traditional teaching style with emphasis on rote learning and its assessment through tests and exams adds to the cognitive demands for the students (Fensham, 2002). These factors dash the hopes of the students who enter the science stream with positive expectations very quickly. The difficulty of the subject matter has been associated to insufficient knowledge foundation (Boujaoude & Barakat, 2000). Too much content to be taught at the first year level in colleges and universities also highlights a need to review the syllabus. Bodner (1992) stated that reviewing the syllabus is not enough; there is a need to rethink the way chemistry is taught. A need to improve upon chemistry learning by considering the course content organization, staff/student relationship and, accounting for students' social and cultural background and learning styles has been proposed (Gabel, 1992; Kirskwood & Symington, 1996). Research studies have shown that if students do not construct an appropriate understanding of the fundamental chemical concepts from the beginning, they cannot learn advanced concepts that are based on the basic ones (Nakhleh, 1992). Students' incomplete and vague answers to examination questions also reflect a lack of understanding of the concepts (Dhindsa & Treagust, 2009). Moreover, there is a need to rethink the way that chemistry is assessed. In the case of multiple-choice tests, students might get the correct response by guessing or by eliminating other choices for a question without understanding the content. Moreover, they may get full mark for partial acceptable knowledge (Dhindsa & Treagust, 2009).
Research studies show that the insufficient knowledge foundation can be the result of many factors associated with the teacher, students and the curriculum, however very little attention has been paid to sustainability of the learned content knowledge. The curriculum developers sequence the content knowledge with increasing difficulty to match with the mental development of the school students as their age increases. Much research in second half of the 20th century concentrated mainly on science content organization (Kempa, 1997). He classified this research into four categories: (a) analysis and appraisal of the science content, (b) selection of concepts and skills to be taught, (c) development of new approaches to different concepts and (d) development of novel learning experience and experiments. He also stated that the focus of the research during late 20th century changed to research on the improvement of the quality of teaching and learning. Despite emphasis changes both types of research still continue. For example, Fensham (2002) recommended that we teaching impact of force on solid bodies in our classes, whereas the social demand is the impact of force on hollow bodies (Accidents with hollow humans in hollow cars). Teachers can assist students to make connections by using carefully sequenced examples, including examples of students' own solution strategies, to illustrate key ideas (Watson & Mason, 2006). According to Anghileri (2006) effective teachers support students to make connections by providing them with opportunities to engage in complex tasks and by setting expectations that they explain their thinking and solution strategies and that they listen to the thinking of others. Criteria for effective teaching also stressed that effective teaching stresses sequence (OPSU, 2010). Despite above concentrated research efforts in both areas especially in science content area, the concept of teaching for sustainable content leaning has attracted very little attention.
However, sometimes the concepts are taught in a simplistic manner that they need to be modified soon after, which requires unlearning of the learned information and relearning of the new information. The new information is superior to the formerly learned information in terms of its ability to explain additional phenomenon. The process of learning, unlearning and relearning is an indicator of our teaching being not for sustainable learning in some instances. Sustainability, in a general sense, is the capacity to maintain a certain process or state indefinitely. Teaching for unsustainable learning not only adds to the students' frustrations, but also is a waste of their and their teachers' valuable time. Moreover, this type of teaching adds to students' misconceptions at the cost of national economy. Teachers mostly rely on the prescribed textbooks to teach content and often teach the topics in the order in which it is arranged in the text book. Some teachers may extend their search to internet to get some relevant information to teach chemistry concepts at school level. In this study three examples have been selected (two examples from the textbooks and one from the internet) to demonstrate how some scientific information is taught at school level and how it can be taught for sustainable learning. These examples include (a) Definition of an element, (b) Electronic configuration of atoms in elements and (c) Volume of two miscible liquids.
Definition of an element is taught to Form 1 students (Median age 12 years) in Singapore and to Form 2 students (Median age 13 years) in Brunei. In this section information associated with definition of an element from two textbooks used in Singapore and Brunei is analyzed for its connectivity, accuracy and sustainability. These textbooks are reported under following sections: Singapore Model and Brunei Model. Recommendations are made to improve the connectivity, and accuracy to teach it for sustainable learning.
(a) Singapore model (Form 1):
Source: "In Science (Vol
1)" (Ho,
et. al, 2009).
This book is recommended for lower secondary science students
in Singapore. Following excerpts associated with definition of
an element have been selected from this book for analysis.
Page 67
An element is a substance which cannot be broken down into simple
substances by chemical reactions. It is therefore the basic building
block of matter.
Page 153
What are atoms?
An atom is defined as the smallest particle of an element that
can exist.
Pages 154-155
Development of the atomic models
Under this section, atomic models are discussed. These include:
Dalton (1803), Thomson, (1897), Rutherford (1911), Bohr (1915)
and Quantum (1926) models. An atom in each model is expressed
in the form of a coloured diagram that follows theoretical descriptions
of postulates of the model. For example, Dalton (1803) model shows
a picture of an atom as a small solid particle. It follows the
postulates of the Dalton's atomic theory:
(Readers can consult source to see the colored pictures; they are not included in the report because of copyright issues).
Page 158
Atoms of the same element contain the same number of protons and
those of different elements contain different numbers of protons.
(b) Analysis of Singapore model
The Singapore model demonstrates a smooth progression for content connectivity, accuracy and sustainability. The definition of an element as "the simplest substance which cannot be broken down into simple substances by chemical reactions" (page 67) is sustainable. It does not require a change. The next statement "It is therefore the basic building block of matter" (page 67) highlights that interaction between elements produces different types of matter.
The description of an atom as "the smallest particle of an element that can exist" connects atoms to the element (not to the matter as is done in Brunei model which is reported in next section). The introduction of various models introduces the subatomic particles. I believe the introduction of quantum model is too advanced for Form 1 students. However, the introduction of electron, proton and neutron as done in the Singapore model has been essential for providing a sustainable definition of an element: "Atoms of the same element contain the same number of protons and those of different elements contain different numbers of protons."
(c) Brunei model (Form 2)
Source: "Secondary Science for Brunei Darussalam (Book 2)"
(Curriculum Development Department, Ministry
of Education, Brunei Darussalam, 2009)
This book is used as a major resource for teaching science in
Brunei Darussalam and most of the teachers follow the order of
content organization during their teaching. Following excerpts
have been selected from this book for analysis.
Page 28
Matter is made up of extremely small particles called atoms. There
are more than 100 different types of atoms. These are of different
sizes. Substances which contain only one kind of atoms are called
elements. Examples of elements given are copper, mercury and gold.
(These elements are also shown in coloured pictures).The descriptions
of these examples are given as stated below.
Copper is an element. It is made up of copper atoms. Gold is an element. It is made up of gold atoms. Scientists put all the elements in a table. This is called the periodic table. From the periodic table, we can find that
Introduction of the electron as a source of electric current without the introduction of subatomic particles on pages 86 and 87.
Page 86: An electric current is
the flow of electrons
An electric current is the flow of electrons in the same direction.
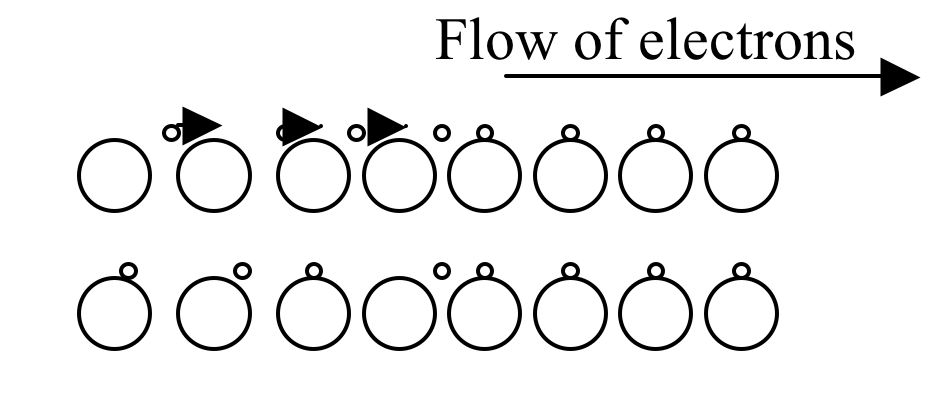
To get an electric current, electrons move from one atom to another
(Figure 1).
; ;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;
;;
;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;
;;
Figure 1 Electric current as the flow of electrons (Modified version of the diagram shown in the text book, but principle is the same
Page 87
A diagram is used to demonstrate the flow of current. A modified
version of the diagram is given in Figure
2.
Figure 2 Electric circuit showing the flow of current.
The description of the diagram follows here after. The electric cell is like a pump. It gives energy to the electrons and pushes them around a circuit. The diagram shows what happens. Note the direction in which electrons move.
An electric current is a flow of electrons from the negative terminal to the positive terminal of a cell.
(Readers can consult source to see the colored pictures; they are not included in the report because of copyright issues).
(d) Analysis of Brunei model
First of all, the first statement that 'Matter is made up of extremely small particles called atoms' on page 28 is unsustainable because it is applicable to simplest type of matter that is elements. Even in some of the elements the molecular state persists. Moreover, matter does not consist of only elements, their interaction produces matter too. For example water is an example of the interaction between one oxygen and two hydrogen atoms. One oxygen and two hydrogen atoms without interaction do not produce water (a different type of matter). Based on similar interactions, matter does exist in the covalent and ionic state. As soon as we start talking about covalent and ionic compounds (matter), the statement 'matter is made up of atoms' requires adjustment.
Also, the statement 'Substances which contain only one kind of atom is called element' is also unsustainable because students do not get a clear picture or understanding of what do the teachers mean by 'same type of atoms'. Actually this statement does not represent science; it is a layman's description of an element. There is no mention of sub-atomic particles. Moreover, as soon as we teach isotopes, the concept of same type of atoms becomes more complex and it needs additional clarification.
Moreover, on page 86 the term electron is introduced as the source of electric current, without prior mention of it. On page 87, movement of electron in a closed circuit is shown diagrammatically.
(e) Summary of Analysis of Singapore and Brunei models
An analysis of the above information suggests that in the Brunei model the teaching is much more unsustainable and weakly connects content knowledge; which is not the case in Singapore model. However, what can we do to improve the Brunei model?
(f) Suggestions to Improve Brunei model
Matter is made up of extremely small particles has already is introduced in Unit 9 and on page 21. It has been classified into solid, liquid and gas. It is possible to introduce another classification of matter as simple and complex; where simple matter is defined as elements with examples such as carbon, iron etc. Then only linking atoms to elements will be easy. However, before giving a sustainable definition of an element in terms of its atoms, it is important to introduce the students with subatomic particles at this stage. A statement that atoms are made up of protons, neutrons and electrons is required. I believe students at this level can understand this much information. It may not be necessary at this stage to teach the location of these particles in an atom, but teaching students that atom is made of protons, neutrons and electrons and different atoms have different numbers of protons, neutrons and electrons is essential. The first part in the above statement will introduce subatomic particles; the second will link to the variations in size of atoms (There are more than 100 different types of atoms. These are of different sizes, Brunei Model page 28). The introduction of protons, neutrons and electrons at this state can be easily achieved. Moreover, since the electron is introduced on page 86, we should introduce it on this page.
Now the questions are (a) do students know the proton, neutron and electron vocabulary and if not (b) how difficult will it be to introduce this vocabulary. In a Form 1 class of 25 students, 18-20 students knew the proton, neutron and electron vocabulary. The teacher can ask the students to the watch Jimmy-neutron TV show and record the names of the characters in the show before teaching this topic. There are many Proton (Malaysian made) cars in Brunei. Linking PEN (a writing pen) to protons, electrons and neutrons can also help. Once this vocabulary is known to students, it is possible to teach a sustainable definition of an element in terms of atoms with same number of protons. It is believed that these simple changes to curriculum sequencing can improve the sustainability of teaching content in the Brunei model.
Under this heading information on electronic structure of atoms in two textbooks one recommended for the GCSE and other for the O-level is analyzed for its connectivity, accuracy and sustainability. Brunei government schools use the O-level textbook. These books are discussed under subheadings GCSE model and O-level model.
(a) GCSE model
Source: "Examining GCSE Chemistry" (McDuell, 1989).
Following paragraph summarizes the way information about the electronic structure of atoms is presented in this textbook.
The Unit 7 on page 41 reports that elements are made of atoms; then sub-atomic particles are introduced followed by atomic number, mass number, arrangement of particles in an atom and isotopes. On page 43 under the heading 'Arrangement of particles in an atom', the term nucleus (consisting of protons and neutrons) is introduced as a positively charged stable (except in case of radio active atoms) part of the atom. Electrons move around the nucleus in energy levels. The maximum capacity of each energy level (1st = 2; 2nd = 8; 3rd = 18 etc) is introduced in diagrammatically. However, the 2n2 model to compute maximum capacity of an energy level is not introduced. The terms K, L, and M are also introduced. Stability of K and L shells with 2 and 8 electrons is introduced. Partial stability of M shell with 8 electrons is also stated: 'However, when eight electrons are in the third energy level it gives it some stability and next two electrons go into fourth energy level. The extra electrons enter the third energy level until it contains the maximum of 18 electrons'. Electronic configuration of carbon-12 is introduced diagrammatically. Electronic configuration of Na and K are reported as 2, 8, 1 and 2, 8, 8, 1. Electronic configurations of first 20 elements are reported on page 44 of text book as reported as Figure 3 in this manuscript.
|
|
(Z) |
(A) |
|
electrons |
||
|
|
|
|
||||
| Hydrogen |
|
|
|
|
|
|
| Helium |
|
|
|
|
|
|
| Lithium |
|
|
|
|
|
|
| Beryllium |
|
|
|
|
|
|
| Boron |
|
|
|
|
|
|
| Carbon |
|
|
|
|
|
|
| Nitrogen |
|
|
|
|
|
|
| Oxygen |
|
|
|
|
|
|
| Fluorine |
|
|
|
|
|
|
| Neon |
|
|
|
|
|
|
| Sodium |
|
|
|
|
|
|
| Magnesium |
|
|
|
|
|
|
| Aluminum |
|
|
|
|
|
|
| Silicon |
|
|
|
|
|
|
| Phosphorus |
|
|
|
|
|
|
| Sulphur |
|
|
|
|
|
|
| Chlorine |
|
|
|
|
|
|
| Argon |
|
|
|
|
|
|
| Potassium |
|
|
|
|
|
|
| Calcium |
|
|
|
|
|
|
Figure 3 Electronic configuration of first 20 elements.
(b) Analysis of above Information (GCSE model)
The analysis of the above reported information revealed that it is relatively well connected and explanations are acceptable. For example, the statement the maximum capacities of K, L and M shells are reported. A partial stability of M shell with 8 electrons is stated. It is also mentioned that 2 electrons will enter 4th shell before 3rd shell completes with 18 electrons. It is demonstrated using Potassium as an example. . The quality of information and its connectivity is better when compared to O-level model described below.
(c) O-Level model
Source: "Chemistry for O- and N-levels; Brunei Darussalam Edition" (Tan et al., 1997).
Following paragraph summarizes the way information about the electronic structure of atoms is presented in this textbook.
The Part A, Unit 6, page 67 of this book stated that elements are made of atoms; followed by the introduction of sub-atomic particles atomic number, mass number, isotopes and electronic structure of particles in an atom. The 'Electronic Structure' sub-topic covers that electrons determine the chemical properties of an element. The electrons travel around the nucleus only in certain fixed shells. Electrons will first occupy the shell nearest to the nucleus; the first shell. The first shell can contain no more than two electrons. When the first shell is completely filled, the remaining electrons will then occupy the second shell and so on. The second shell can contain no more than eight electrons. The 1st, 2nd, and 3rd shells are introduced using example of Sodium atom. These descriptions follow the examples of electronic structures of H, He, Li, Be, Na and K (see pages 67-69). The next sub-topic: 'Electronic Structure and the Periodic Table' where the number of filled shells and the number of electrons in the outmost shell are related to period and group in the periodic table. On page 71, part of the periodic table is reported for first 20 elements. The table is reported as Figure 4 in this manuscript.
|
|
|
|
|
|
|
|
|
|
H 1 |
He 2 |
||||||
|
Li 2,1 |
Be 2,2 |
B 2,3 |
C 2,4 |
N 2,5 |
O 2,6 |
F 2,7 |
Ne 2,8 |
|
Na 2,8,1 |
Mg 2,8,2 |
Al 2,8,3 |
Si 2,8,4 |
P 2,8,5 |
S 2,8,6 |
Cl 2,8,7 |
Ar 2,8,8 |
|
K 2,8,8,1 |
Ca 2,8,8,2 |
||||||
Figure 4 Electronic configuration of first 20 elements (Adapted from Tan et. al, 1997; The table in this book also shows diagrammatically the distribution of electron in energy levels for each element).
(d) Analysis of above Information (O-Level model)
The analysis of the above reported information (used in Brunei) is weakly connected and explanations are not complete. For example, the statement "When the first shell is completely filled, the remaining electrons will then occupy the second shell and so on" assumes that reader will know how many electrons are filled in 3rd shell. No mention of filling of 2 electrons in 4th shell before filling the 3rd shell completely. Information presented lack explanations and reader is expected to rote learn. The quality of information and its connectivity is poorer when compared to GCSE model.
(e) Problems with Information in both Textbooks (GCSE and O-level models)
The introduction of the electronic configuration using above style faces some serious problems. The models did not introduce the sustainable mathematical function the 2n2, (n = number of shell, n value for K=1, L = 2, M = 3 and so on) for students to calculate the maximum number of electrons in each energy level (2 in 1st; 8 in 2nd; 18 in 3rd, and so on). Argon is demonstrated to be stable with incomplete 3rd shell. Moreover, the information given in Figures 3 and 4 give an impression that the addition of electron in the fourth period too will continue till it gets to 8 as filled. Based on author's experience, students expect electronic configuration of scandium (21) to be 2, 8, 8, 3; that is not true. Hence, our teaching has to undergo the process of un-teaching and re-teaching as soon as we need to deal with atomic number 21. Hence this teaching is not sustainable. Moreover, students get frustrated. In order to overcome the above problems, the model of 2, 8, 8, 2 that causes more confusion should not be taught and also I strongly recommend that we should not limit teaching electronic configuration to first 20 elements.
Figure 5 A mechanical model to determine electronic configuration of an atom.
(f) Proposed Solution
I propose that after teachers have introduced shells, they should introduce the 2n2, function to determine the maximum electron capacity of the shells. To know the electronic structure they should introduce the mechanical model (staircase model; see Figure 5). The students at O-level can easily draw it. Starting from top right corner, they should draw an arrow diagonally till it touches the front line. Then from every bend of the stair they should draw similar arrows diagonally as shown in the Figure 5. Link the boxes in the rows to shells and the total number of electrons in boxes in a row will give actual number of electrons in the shell. Teacher should also introduce the students that maximum capacity of boxes in columns one is 2 and of second is 6. Why 2 and 6 electrons, we can tell the students that you will learn this next year. The filling starts from tail of the arrow in the top box. When the student reaches the arrow, he/she moves to the next arrow's tail and shall continue till all electrons are filled. Then electrons in each shell are added under the column 'Actual electrons #'. Give some examples as given Figure 6 and Appendix.
Electrons in Aluminum = 13 and electronic configuration: 2, 8, 3
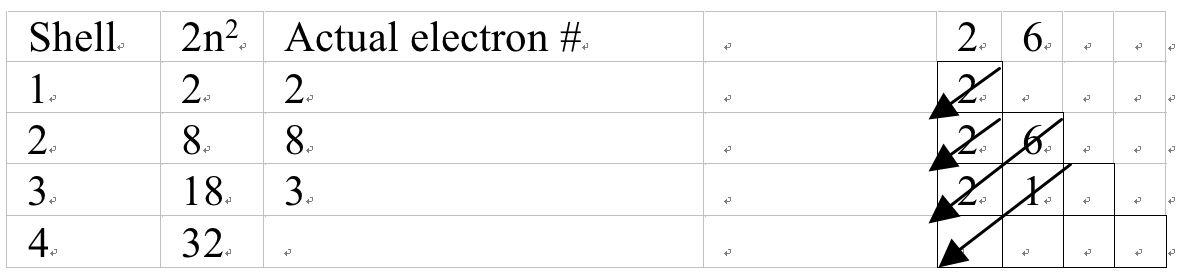
Figure 6 A mechanical model to determine electronic configuration of an atom.
Aluminum has 13 electrons, 2 goes into the top box leaving behind 11. Then 2 are filled in the box with second arrow; leaving being 9 electrons. The third arrow extends in two boxes. Out of 9 electrons 6 of then are put into the tail box and 2 in the box with arrowhead; leaving behind 1. The last electron enters the tail box for the fourth arrow. Then electrons are added in each row; thus giving 2, 8 and 3 electrons on 1st, 2nd, and 3rd shells respectively.
This is simply as mechanical model to compute the number of electrons in a shell. It is easy to draw and comprehend. Once students have learned it, during the following year they can extend it to elements with higher atomic numbers. The learning of this model at O-level is sustainable; saves teaching time and students from frustrations at A-level. It can be successfully linked to 2n2 formula taught earlier. It can help to introduce sub-energy levels and shells (s, p, d, and f sub energy levels), their electronic capacity during A-level. It will help students learn quantum model that explains existence of sub-energy levels beyond f sub-energy level.
Source: "Miscibility of Fluids" (Helmenstine, nd)
Problem:
If you add 50 mL of water to 50 mL of water you get 100 mL of
water. Similarly, if you add 50 mL of ethanol (alcohol) to 50
mL of ethanol you get 100 mL of ethanol. But, if you mix 50 mL
of water and 50 mL of ethanol you get approximately 96 mL of liquid,
not 100 mL. Why?
Anne's response:
The answer has to do with the different sizes of the water and
ethanol molecules. Ethanol molecules are smaller than water molecules,
so when the two liquids are mixed together the ethanol falls between
the spaces left by the water. It's similar to what happens when
you mix a liter of sand and a liter of rocks. You get less than
two liters total volume because the sand fell between the rocks,
right? Think of miscibility as 'mixability' and it's easy to remember.
Fluid volumes (liquids and gases) aren't necessarily additive.
Intermolecular forces (hydrogen bonding, London dispersion forces,
dipole-dipole forces) also play their part in miscibility, but
that's another story.
Analysis and proposed solution to the problem:
A major problem with this model is that students cannot think that H2O is bigger than C2H5OH. It is not clear if Anne was thinking of cluster of water molecules as a result of hydrogen bonding. It does not account for the polarity of the molecules. Also, there are differences in molecular sizes, shapes and functional groups; hence some parts of one molecule can enter the gaps between others. Moreover the molecules of solvent and solute are always in motion. It means the volume of the mixture should always decrease. This is not the case. The volume can be equal to or more than the mathematical sum. Hence the use this model to explain the volume changes is not sustainable. It will require a correction.
Proposed Solution
Considering that two types of molecules in a setting arrange in a way to minimize repulsions, I propose that the attraction between two types of molecules should be used as an explanation. When the force of attraction between two different molecules is about the same as they have between two of their own kind, the mixture volume will add up mathematically. When the force of attraction between two different molecules is greater than that they have between two of their own kind, the volume will be less than the mathematical sum. When the force of attraction between two different molecules is smaller than that they have between two of their own kind, the volume will be more than the mathematical sum. This explanation is sustainable. Moreover, we can use analogy of Love and Hate between two students.
We all have some private space around us. When we love someone, we can share this space with him/her. When these two persons come close to each other, an overlap of the private spaces of the two persons occurs as a result the total space around the two persons is now less than the sum of the spaces of the two persons. This will represent an attraction between two different molecules. However, if we hate some one, we do not like to go even close to him/her. Even touching the boundaries of these two private spaces does not occur. Under this case the total private space around two people will be more than the total sum of private spaces of two people. It will represent when the force of attraction between molecules is low and overall volume increases. There is no sharing of private space with unknown persons. It will represent a case when force of attraction between molecules in mixture is not different from that of their own kind. In this case the volume of mixture will add up mathematically.
The above examples show that our classroom teaching of many concepts is not sustainable. Small changes to the curriculum could help us to make the teaching sustainable. Sustainable content teaching will save teaching time and also avoid the time used for learning, unlearning and relearning. Moreover, it will minimize the students' frustrations. The teachers once get the habit of thinking about how to teach content for sustainable learning; they will be applying this knowledge whenever they are to teach new knowledge. The curriculum department can benefit by revising the curriculum to minimize the gaps in the sequence of science topics to be taught in different levels, at a level and in a lesson.
The scientific knowledge is growing exponentially. As a result, more complex knowledge is moved to school curriculum for learning. Considering the mental capacities of secondary students, learning of the large quantity of complex knowledge is not possible in the time available to them. One of the possible solutions to minimize the above problem is to teach for sustainable learning by minimizing learning, un-learning and relearning cycle. Hence it is time for the international scientific community to decide what scientific knowledge/models we shall teach to improve sustainable learning.
Electronic configurations of H, C, Ar and K
Electrons in Hydrogen = 1 and electronic configuration:
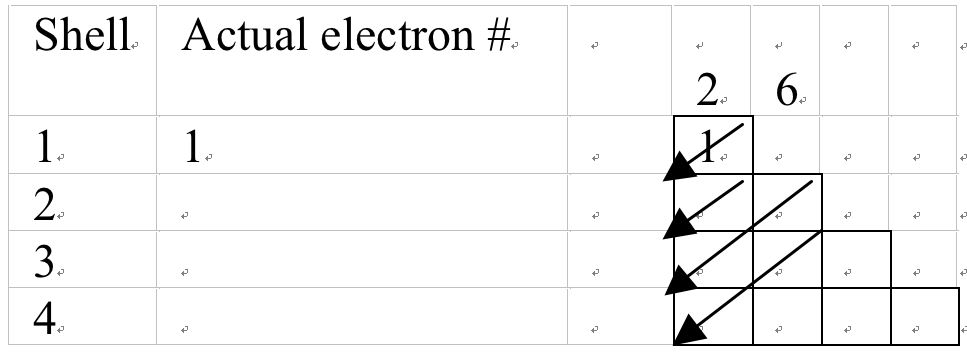
Figure 6a Using the Mechanical model to determine electronic configuration of Hydrogen.
Electrons in Carbon = 6 and electronic configuration: 2, 4
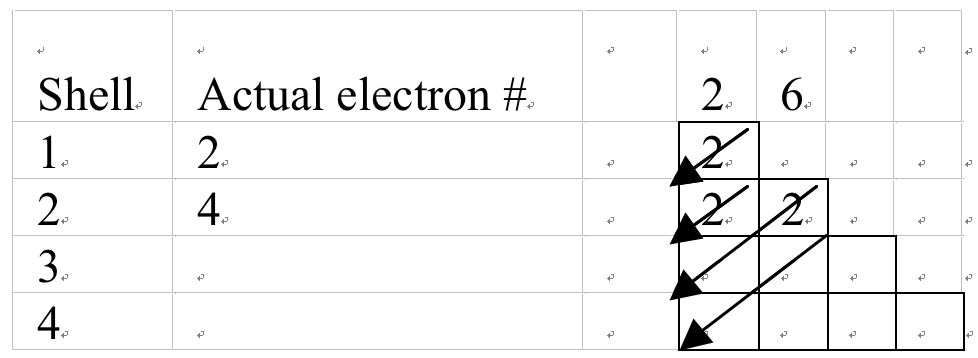
Figure 6b A Mechanical model to determine electronic configuration of carbon.
Electrons in Argon = 18 and electronic configuration: 2, 8, 8

Figure 6c A Mechanical model to determine electronic configuration of Argon.
Electrons in Potassium = 20 and electronic configuration: 2, 8, 8, 2
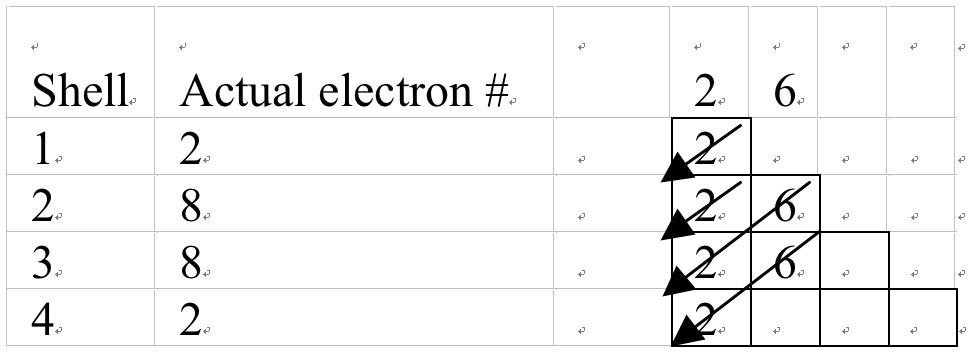
Figure 6d A Mechanical model to determine electronic configuration of Potassium.