Chemical Education Journal (CEJ),
Vol. 14, Issue 1 /Registration No. 14-5 /Received June 11, 2011.
URL =
http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
Teachers can highlight the application and importance of a particular
topic, to make courses more meaningful. Polymers are a part of
our daily life. This article elucidates why students should take
interest in 'polymer viscosity'. Some viscosity based nomenclatures
are introduced at the beginning. It is being shown how some practically
important parameters such as molecular weight of polymer, average
end-to-end distance of polymer and degree of branching in polymer
can be derived from the intrinsic viscosity values. Some interesting
experiments are suggested at the end, which can nurture curiosity
and critical thinking among students and improve correlation between
courses and real world things.
Keywords: polymers; intrinsic viscosity; Flory-Fox theory; end-to-end distance; radius of gyration; molecular weight; Ubbelohde viscometer; waste plastic bottles; real world application; critical thinking; comprehension; enthusiasm
Classification based on viscosity
Average end-to-end distance of polynmer molecule
Degree of branching of the polymer
Measurement of viscosity and proposed experiments
'Polymer' is included in Physical Chemistry courses in India and Europe, whereas in USA it is considered as a part of Organic Chemistry. These categorizations are just to simplify and organize courses and as we all know, when it comes to research, these discipline-based barriers vanish very quickly.
Irrespective of this debate regarding which discipline it should belong, 'polymer' is an integral part of our daily life, no doubt. Students sometimes fail to see the relevance of chemistry and can not connect their lessons with real world things. Highlighting the application and importance of a particular topic during teaching, can make courses more meaningful and invoke enthusiasm.
One of the fundamental properties of polymer solution is its 'viscosity'. Intrinsic viscosity, [η], of a polymer solution is expressed as follows:
[η] = lim [η]red = lim [η]sp / c ······ (1) aaaaa c→0 aaaaaaaac→0
where reduced viscosity, [η]red, is equal to specific viscosity divided by concentration of polymer solution and specific viscosity,
[η]sp = [η]rel - 1 ······ (2)
Relative viscosity, [η]rel is again equal to η / η0, where η and η0 are the viscosities of polymer solution and pure solvent respectively. Apparently the unit of intrinsic viscosity is inverse of concentration unit e.g. dL/g.
Viscosity is the measure of resistance of a fluid to flow. Water has a viscosity of 1 cps (centipoise) at room temperature and is considered as a standard. Newtonian fluids have a viscosity which is dependent only on temperature, not on shear rate and time e.g. sugar solution. On the other hand, depending on how viscosity changes with time, the flow behavior of non-Newtonian fluid can be categorized as: (a) thixotropic (time thinning, i.e. viscosity decreases with time) e.g. yoghurt and (b) rheopetic (time thickening, i.e. viscosity increases with time) e.g. gypsum paste.
The viscosity of a Non-Newtonian time independent fluid is dependent not only on temperature but also on shear rate (Figure 1). Depending on how viscosity changes with shear rate, the flow behavior is classified as:
Sear thinning fluids are also called pseudoplastic and shear thickening fluids are also called dilatant.
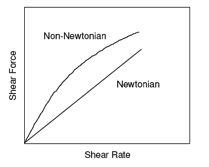
Figure 1 Schematic plot of shear force vs. shear rate for Newtonian and Non-Newtonian fluids.
One can find the above-mentioned terminologies and expressions in any standard textbook of polymer chemistry. But why should students really want to know about polymer viscosity? Why is it important? Because it offers a wealth of information about the polymer sample. What are those? Three important parameters can be derived from intrinsic viscosity data of a polymer solution - (a) molecular weight of polymer, M (b) average end-to-end distance of polymer molecule, <h2>1/2 and (c) degree of branching of the polymer, g.
Mark-Houwink equation [1] gives a relationship between intrinsic viscosity, [η] and molecular weight, M of a polymer solution, expressed as:
[η] = KMa ······ (3),
where the constants, a and K, depend on the particular polymer-solvent system. A value of a = 0.5 is indicative of 'theta solvent'. A value of a = 0.8 is typical for 'good solvents'. For most flexible polymers, 0.5 ≤ a ≤ 0.8. For semi-flexible polymers, a ≥ 0.8. For polymers with an absolute rigid rod, such as Tobacco Mosaic Virus, a = 2.0.
Averaging of polymer molecular weight is required, as polymer is a mixture of various molecular weights and molecular sizes. Depending on the experimental techniques used, polymers may have four different kinds of average molecular weights [1]: (a) weight average molecular weight, Mw (light scattering) (b) number avergae molecular weight, Mn (colligative properties) (c) Z-average molecular weight, Mz (centrifugation data) and (d) viscosity average molecular weight, Mv (viscometric behavior) and the relative order of their values are: Mz > Mw > Mv > Mn (Figure 2). Molecular weights based on viscosity measurements are less precise, as it depends on the solvent used, but is less expensive and easy to perform.
Figure 2 Four average momlecular weights of polymer
According to Flory-Fox theory [2], the intrinsic viscosity [η] is given by:
[η] = φ<h2>3/2 / M ······ (4),
where [η] is the intrinsic viscosity of a polymer solution in a given solvent and at a given temperature, <h2>1/2 is the average end-to-end distance of the polymer molecule in a given solvent and at a given temperature, M is the molecular weight of the polymer and φ is universal viscosity constant independent of the polymer-solvent system, the most acceptable value being 2.5x1021 mol-1 for flexible and hetero-disperse polymers in good solvents. Hence after determining [η] and M experimentally, it is possible to calculate <h2>1/2. Flory-Fox theory assumes a flexible polymer molecule as a non-draining coil and in an 'ideal' solution it can be approximated to a hydrodynamic sphere.
Consider a polymer chain as an assembly of mass elements (structural units) of mass mi, each located at a distance ri from the center of mass of the molecule. By definition, the radius of gyration at a particular configuration is given by:
<RG>2 = <∑ miri>2 / mi.
Mean-square end-to-end distance, <h2>, is a value which is meaningful only for perfectly linear polymers having two ends. Macromolecules, which do not have a free end (e.g. macrocycles) or have several free ends (e.g. branched polymers, star polymers etc) can not be described by end-to-end distance. It is more appropriate to use mean-square radius of gyration, <RG>, as a measure of chain dimension. The inter-relation is as follows, derivation of which is beyond the scope of this text [3]:
<h2> = 6<RG>2 ······(5),
Hence using equations (4) and (5),
[η] = φ.6 3/2.(<RG>2)3/2 / M = φ'.(<RG>2)3/2 / M,
where φ' = φ.6 3/2, or
<RG>2 = [η] M / φ' ······(6)
The degree of branching (g) is defined as:
g = <RG>2 (branched) / <RG>2 (linear)······(7)
where the suffixes (branched) and (linear) stands for the branched and linear polymers respectively, having same molecular weights and same number of repeat units.
Hence for a given polymer having same 'M', one can obtain from equations (6) and (7),
g3/2 = [η] (branched) / [η] (linear).
[η] can be determined experimentally for both branched and linear chain polymers, which in turn will give 'g', provided the polymers have the same molecular weight.
Viscometers can be of three types: (a) to measure kinematic viscosity e.g. Ubbelohde viscometer (b) to measure rotational viscosity e.g. Brookfield viscometer and (c) to measure dynamic viscosity e.g. controlled stress rheometer from TA Instruments.
Simple experiments can be designed to ignite curiosity, nurture critical thinking and provide hands-on experience among students. An interesting experiment will be to ask the students to bring different types of waste plastic bottles (also a good opportunity to introduce different plastic recycling symbols often used in consumer goods: Figure 3 [4]), ask to measure the corresponding viscosities and hence calculate the respective molecular weights of the polymers and identify different polymers based on molecular weight.

Figure 3 Common plastic recycling symbols
Most of the bottles for carbonated drinks, mineral water, edible
oil and personal care products are made of poly(ethylene terephthalate),
PET and their intrinsic viscosity values range from 0.7-0.85 dL/g,
depending on the length of the polymer chain (the longer the polymer
chains, the more entanglements between the chains and therefore
the higher the viscosity).
Students should prepare sample solutions according to ASTM
D4603-03 standard test method [5].
This test method is for the determination of inherent viscosity
of PET soluble at 0.50% concentration in a 60/40 phenol/1,1,2,2-tetrachloroethane
solution by means of a glass capillary Ubbelohde viscometer. A
50/50 by weight mixture of phenol and 1,2-dichlorobenzene will
work as well. The polymer is first weighed and then dissolved
in the solvent, as mentioned above. The solution and the viscometer
are placed in a constant temperature water bath, until thermal
equilibrium is obtained. Time required for 100 ml of this solution
to flow through the capillary viscometer from the upper to the
lower graduation mark (Figure 4) at
the fixed temperature, is noted down which is proportional to
the viscosity of the sample solution, η. Students will repeat
this experiment for five different concentrations of polymer solution.
Also the flow time for the solvent under the same conditions is
noted down, which is proportional to the viscosity of solvent,
η0.
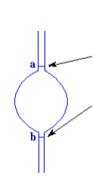
Figure 4 A portion of Ubbelohde viscometer showing the upper and lower graduation marks, 'a' and 'b' respectively.
Concentration and times are then used to calculate the reduced viscosity of the polymer solution using equations (1) and (2). A plot of reduced viscosity vs. concentration will produce a straight line and extrapolating the straight line to zero concentration, the intrinsic viscosity of the polymer solution can be obtained from the y-intercept (Figure 5). Molecular weight of the polymer will be calculated thereafter using equation (3).
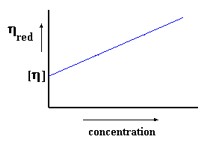
Figure 5 A plot of reduced viscosity vs. concentration of a polymer solution, y-intercept corresponding to intrinsic viscosity
In conclusion, this article shows some viscosity-based nomenclatures, how a wealth of information can be derived from polymer intrinsic viscosity, and proposes some lab experiments to ignite enthusiasm and better comprehension among students.
The author is thankful to her mother, P. Dey, who, having a background in science as well as in education, has been an inspiration to the author.