Abstract
An experiment in UV-spectroscopy as part of Discovery chemistry
curriculum is described in this article. Discovery Chemistry is
a pedagogical philosophy that makes the laboratory the key center
of learning for students in their first two years of undergraduate
instructions in some of the universities in America. Questions
are posed in the pre-laboratory discussion and assessed using
pooled student data. Results of experiments are then used in lectures
to discover chemical principles. This pedagogy philosophy can
be extrapolated to senior undergraduate or graduate students across
other universities in the world. This experiment enables the students
to record the time-dependent spectra, evinces interest in the
interpretation of isosbestic point and assists in comprehending
the intricacies of the mechanism of solvolysis of (dichloromethyl)benzene.
Further it is a prelude to hone their research skills (searching
for the answers in the references, i.e. research articles).
Keywords: Senior undergraduate or graduate students, UV spectroscopy, Inquiry-Based, Discovery Learning
Contents
The study of isosbestic point is a part of undergraduate curriculum in Mizan Tepi University (Ethiopia) and other universities. Usually different forms (acid form and basic form) of Bromocresol green are taken for the demonstration of isosbestic point. In this article we have introduced a simple experiment by virtue of which the students can very easily observe two distinctly visible isosbestic points. The experiment is followed by some extremely pertinent questions, the answers of which would lead to the conceptual comprehension of isosbestic point, assists in comprehending the intricacies of the mechanism of solvolysis of (dichloromethyl)benzene and to acclimatize the students to the alternative interpretation1 of isosbestic point.
(Dichloromethyl)benzene, HPLC grade acetonitrille, Millipore distilled water, Micro pipette, Double beam spectrophotometer of the type UVIKON-923 (from Milan).
All the chemicals used in this experiment are non-hazardous to the students.
A stock solution of (dichloromethyl)benzene of concentration 1 x 10-2 M was prepared in HPLC grade acetonitrille. From this stock solution 30 mL of the compound was taken and injected into 3 ml of water contained in a spectrophotometric cell, the lid of the cell was placed and the solution was shaken well (a reference solution of 3 ml of water with 30 mL of acetonitrile was taken). Immediately after this, the spectrophotometric cell was placed in the spectrophotometer and the time-dependent spectrum was recorded (in the time interval of about two minutes). This experiment was done to estimate the lifetime of intermediate carbocation2 by the physical chemistry MSc 2nd year students of Osmania University in the year 2003-2004.
The spectrum as shown in Figure 1 was obtained (On the y-axis we have absorbance and on the x-axis we have wavelength in nm). Two distinctly visible isosbestic points were observed in the time dependent spectra of solvolysis of (dichloromethyl)benzene (Figure 1). The spectrum of the expected benzaldehyde was measured under the same concentration as the reactant and it was found that the spectrum was congruent with that of reactant concentration, reflecting that the reaction was complete.
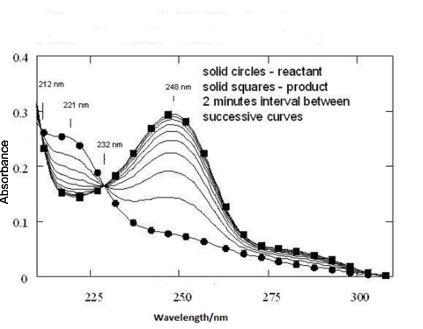
Thus, an extremely simple experiment for the demonstration of isosbestic point(s). In fact the experiment is so simple that this experiment can surrogate any of the experiments which are demonstrated at undergraduate or graduate level for the appearance of isosbestic point.
The mechanism for the solvolysis of (dichloromethyl)benzene is depicted in Scheme 1.2,3
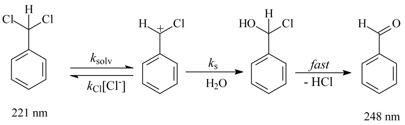
Some cardinal questions which would aid the conceptual comprehension of the isosbestic point are given below. To answer these questions, the students have no alternative but to refer to the references provided. Thus, this exercise of extracting answers from the references is prelude towards formal training in research.
1) What is the reason for the absorbance to be constant at isosbestic point? Give the answer from the stand point of conventional definition of isosbestic point4 and from stand point of alternative interpretation of isosbestic point.1
2) How would you rule out the possibility of the reaction of acetonitrille (1% acetonitrille is present in our system) with a-chlorobenzyl carbocation, i.e. Ritter reaction.2,3,5
3) Predict the number of components at the isosbestic point from the alternative interpretation of isosbestic point,1,6 in addition to conventional definition of isosbestic point.4
4) A similar time dependent spectrum was recorded for (dibromomethyl)benzene7 but when the time dependent spectra for the (diacetoxymethyl)benzene (or benzylidene diacetate) was endeavoured to be recorded, the solvolysis of (diacetoxymethyl)benzene did not take place at all. What are the plausible reasons for the reluctance of (diacetoxymethyl)benzene to undergo solvolysis?8
5) Under what conditions would the solvolysis of (diacetoxymethyl)benzene occur?9
6) How is the unequivocal presence of the intermediate a-chlorobenzyl carbocation in the reaction mechanism established?2,3
For the appearance of the isosbestic point at 233 nm the conventional
definition of isosbestic point4
and the alternative interpretation1
are applicable.
The second isosbestic point at 212 nma)
suggests that there is possibility of second intermediate. The
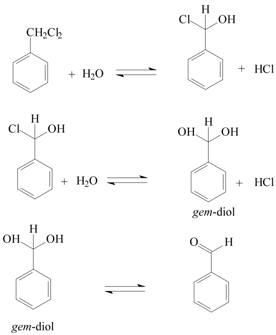
second intermediate might be because of the presence of gem-diol
or hydrated benzaldehyde (Scheme 2).
If at all gem-diol or hydrated benzaldehyde is present,
it is too short-lived to be detected by the spectrophotometer.
But, we do not have any evidence. In fact the empirical explanation
for the presence of the second isosbestic point is an unexplored
territory and the challenge is in front of us.
Acknowledgement: Rachuru Sanjeev (one of the authors) would like to dedicate this manuscript to his late mother, Rachuru Vijaya, a conscientious teacher who saw to it that "the nature and nurture" that he received groomed him into a teacher with the same kind of quality.
a) Most organic compounds including acetonitrile have strong absorption around 215 nm and lower wavelengths, and may cause "blackout" of spectrophotometer in this wavelength range. Close to the blackout range, some spectrophotometers may show some unusual behaviour. Thus, although there certainly exists an isosbestic point observed at 212 nm, the signals may or may not be caused by the solutes themselves in the range below 215 nm. This bleak possibility cannot be ruled out. But, this did not take place in our case. Our instrument never went "blackout".
The reason for the absorbance at isosbestic point to be constant
is that benzaldehyde and (dichloromethyl)benzene, i.e.
the two substances absorb light of that specific wavelength to
the same extent, and the analytical concentration remains constant.
The foregoing sentence offers an explanation for the presence
of isosbestic point from the stand point of conventional definition.
At the isosbestic point, the intermediate a-chlorobenzyl
carbocation is present. In compliance with the steady state principle
the concentration of the intermediate is constant (for the intermediate
concentration to be constant the essential criterion is ks/ksolv > 10).
Since, the concentration of the intermediate is assumed to be
constant, it follows that the absorption does not change with
time (a trivial point to be remembered is that the absorption
is the index of concentration). Hence the absorption is constant
at the isosbestic point.
The foregoing paragraph offers an explanation for the presence
of isosbestic point from the stand point of the alternative interpretation.1
In the reference 3 one has to go to page 52, lines 5-9 and page 53, lines 1-2. The good fit to equation 1 (in reference 3), and the fact that the chloride inhibition effect is taking place with increasing concentration of chloride ion, one can categorically state that the a-chlorobenzyl carbocation is formed during the solvolysis of (dichloromethyl)benzene.