Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-101/Received September 12, 2013.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
The science of chemistry has witnessed a renaissance over
the past century. With the explosion of chemical knowledge, it
is time to consolidate all the information we have amassed and
to rethink how chemistry should be taught to younger generations
at all levels of education from secondary school to college and
the university. Clearly, new textbooks should be written to reflect
all the discoveries and progress made, but greater emphasis should
be placed on the challenging task of structuring the material
around fundamental principles over the presentation of factual
information. As a discovery science, chemistry remains an experimental
discipline, so an integrated laboratory/research proficiency that
provides hands-on experience as well as opportunities for exploratory
activities should go hand-in-hand. Toward improving the quality
of the human resources in chemistry, there is need to update the
chemistry undergraduate curriculum at the university to motivate
bright and creative young people in the intellectual excitement,
career opportunities, and financial rewards of the chemical profession
in order to engage the best minds to join us to meet the challenges
of modern chemistry. As chemical educators, not only do we have
the responsibility to mentor our students so that they can achieve
their intellectual potentials and professional aspirations, but
we must also serve as their role models and impart the leadership
qualities, social responsibilities, and human values that can
help them to educate the general public on the role played by
science and technology in a modern society. Indeed, there are
abundant opportunities for innovation in chemical education during
these changing times.
Keywords: chemical education; curriculum reform; chemistry as the central science; basic chemistry; discovery science.
The events that have tarnished the image of chemistry.
Chemical education is at a crossroad.
Basic chemistry is still very much a vibrant science!
The need to engage the changing student.
Some suggestions on how to improve the quality of the undergraduate education in chemistry.
The past century has witnessed a renaissance in the science of chemistry. Starting from discovery of the elements, we have learned how molecules are formed, and how molecules react to rearrange the atoms to form new molecules. We have developed methods to synthesize them in the laboratory. We also have in hand the tools that can predict new molecules, their properties, and even their chemical reactivity. The field has grown and mushroomed, and eventually chemistry has had to be divided into specialties and sub-disciplines.
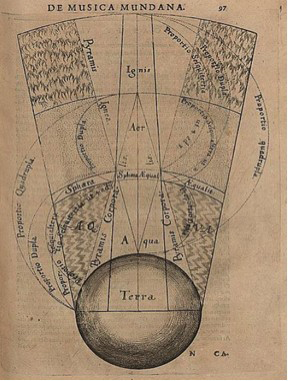
The field of chemistry is moving on, but it is presently experiencing some sort of an image and identity crisis, largely a fall out of our own successes. For several decades now, the general public has had a rather negative perception of chemistry. Not only does the average layman on the street no longer able to relate to chemistry, but also many people blame chemistry for many of the woes of society, including cancers, environmental hazards, global warming and climatic change. Yet, chemistry has to be one of our "closest friends" as we live in a world of molecules. We live on a planet of chemicals whether we like it or not. After all, chemistry started with "earth, water, air, and fire"(Figure1) [1]. Nevertheless, it has only been the past century that scientists have succeeded in creating new molecules that have altered our quality of life, and even changed our lifestyle, in a significant way.
 Click for larger
image.
Click for larger
image.Figure1. The classical elements in Babylonia: Segment of the macrocosm showing the elemental spheres of Terra (earth), Aqua (water), Aer (air), and Ignis (fire). Robert Fludd. 1617. Reproduced from [1].
Most of you are probably too young to have heard of the DuPont slogan "BETTER THINGS AND BETTER LIVING THROUGH CHEMISTRY"(Figure 2) [2]. The DuPont Company is the largest chemical company in the US, and it has done more than any other US corporation, except perhaps Ford and General Motors, to improve our style of living. In 1935, the DuPont Company adopted this slogan in the advertisement of their products, and it was their slogan until 1982 when the "Through Chemistry" part was dropped. Since 1999, the slogan has been updated to "Miracles of Science". Nevertheless, the DuPont Company has had every reason to be proud of their accomplishments, as inventor of neoprene, nylon, Teflon, Mylar, Tyvek, and many other polymers and plastics during the 20th century. DuPont has also developed chlorofluorohydrocarbons (freons) for the refrigeration industry, and later more environmentally friendly refrigerants.
 Click for larger
image.
Click for larger
image.Figure 2. Slogan of the DuPont Company (1935-82): "BETTER
THINGS AND BETTER LIVING THROUGH CHEMISTRY". Reproduced from
[2].
Aside from DuPont, there have been many other chemical companies
that have impacted our daily lives, including BASF, Chrevon Oil,
Dow Chemical, Eastman Kodak, Exxon Mobil, General Electric, Monsanto,
3M, Rohm & Hass, Shell Oil, Sigma-Aldrich, Union Carbide,
just to name a few. Union Carbide introduced polyethylene into
food containers and toys; ethylene glycol into automotive antifreeze,
and isopropanol into rubbing alcohol. From Dow came Styrofoam,
Saran wrap, Ziploc bags and even Scrubbing Bubbles, and other
consumer products that we now more or less take for granted. Eastman
Kodak is best known for its photographic film products.
During the 1950s, 60s and 70s, these companies collectively provided the employment opportunities for many of our graduates in chemistry and chemical engineering. In fact, a career in these companies was highly sought after by our Ph.D.s in those days. Compensations and fringe benefits were more lucrative than academic salaries in the universities, except perhaps in the top chemistry and chemical engineering departments. There were also ample opportunities for advancement to management positions within these industrial companies. Our students were well prepared for industrial research and the company they joined would give them more or less a free hand to initiate R&D programs as soon as they walked in the door. Not surprisingly, they accounted for many of the inventions and products of the chemical and pharmaceutical industries. Job satisfaction was high among these students who opted to work for industry then.
But then came the environmental impacts of the herbicide DDT (Figure 3A) on ecology and related effects on human health [3];the link of certain human cancers and birth defects to dioxin (Figure 3B); the association of the "morning sickness" pill thalidomide (Figure 3C) with deformed children; increases in air and soil lead levels from the use of tetraethyllead (Figure 3D) in gasoline, leading to neurotoxicity and acute lead poisoning; the contribution of freons (Figure 3E) to the depletion of ozone in the earth's stratosphere[4] (Figure 4A); Union Carbide's Bhopal disaster in India in 1984 (Figure 4B), where 40 tons of methyl isocyanate (Figure 3F) leaked into a highly densely populated area; and Exxon's Valdez Oil Spill of Prince William Sound, Alaska in 1989 (Figure 4C). Largely because of these events and others, the image of chemistry became tarnished. Today, we seldom hear that chemistry has done this or that for the benefit of society in the public press. When the news media discuss sequencing of genomes, vaccines, cholesterol-lowering statins, viagra, a new drug for type II diabetes, etc., these accomplishments are always billed as products of biotechnology. But these developments and advances all involve chemistry. So chemistry is still very much alive!!
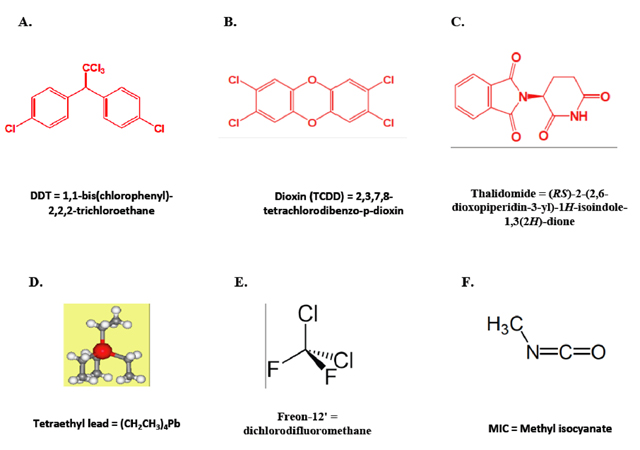
Figure 3. (A) DDT, a highly chlorinated insecticide that bioaccumulates in birds, causing egg-shell thinning, nesting failures and dramatic population declines of ospreys, eagles and brown pelicans. (B) Dioxin, an unintentional by-product of many industrial processes involving chlorine such as waste incineration, chemical and pesticide manufacturing and pulp and paper bleaching, which has been linked to breast cancer, severe reproductive and developmental problems, and birth defects. (C) Thalidomide, the "morning sickness" pill used by women in Europe to lessen the effects of nausea and vomiting during pregnancy, led to ~10,000 children born world-wide with acute birth defects including missing and grossly deformed limbs. (D) Tetraethyllead, an antiknock additive for gasoline to control octane ratings in automobiles, increases air and soil lead levels leading to lead poisoning and accumulative neurotoxicity lowering the IQ's in children. (E) Freon-12', a chlorofluorocarbon (CFC) gas used as a refrigerant and aerosol propellant implicated in ozone depletion in the earth's stratosphere. (F) MIC, an intermediate chemical used in the production of carbamate pesticides, rubbers and adhesives, shown to be a highly toxic and irritating material, and extremely hazardous to human health.
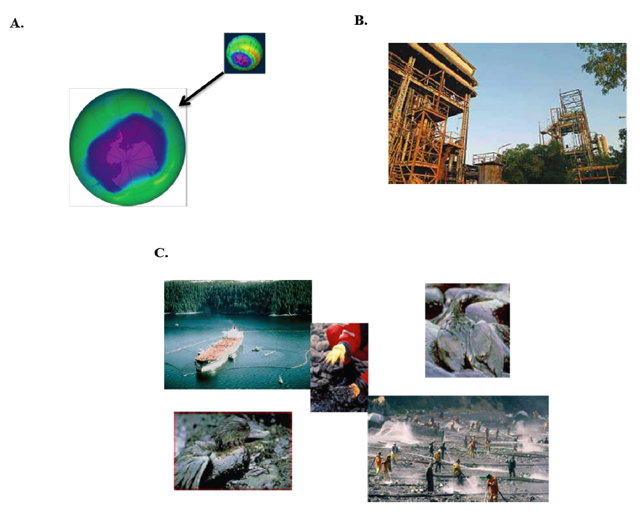
Figure 4. (A) Image of the large Antarctic ozone hole recorded over the Southern pole during September 2006. Chlorofluorocarbons (CFCs), like Freon-12', contribute to the depletion of ozone in the earth's stratosphere (the ozone layer). (B) The Union Carbide India Limited pesticide plant at Bhopal, India, where 40 tons of MIC leaked from underground reservoirs into a densely populated area on December 3, 1984, killing nearly 8,000 initially and approximately 20,000 to 30,000 people in total. (C) The Exxon Valdez oil spill in Prince William Sound, Alaska, on March 24, 1989, when Exxon Valdez, an oil tanker bound for Long Beach, California, struck Prince William Sound's Bligh Reef, spilling 260,000 to 750,000 barrels of crude oil over the next few days.
In response to these tragedies, the government soon instituted laws and regulations to safeguard the public from chemicals and the products derived from chemical processes. The course of development of a product from invention/discovery at the research bench to the consumer market became more intricate as the process faced more and more stringent government regulations to protect the environment as well as the worker. Since the 1980's, industrial R&D has become more and more complex and costly. Eventually, R&D became no longer cost effective and the chemical companies began to scale back on basic research and eventually phased out these activities. Nowadays, most chemical companies farm out basic research to the universities, or simply buy out smaller companies to add new products to their business portfolios if they find something interesting. I do not believe that this situation has changed much the past 20 years despite the improved business climate of the chemical industry.
In more recent times, basic and applied research carried out by the industrial chemist is done mostly in the pharmaceutical houses and Biopharms like Amgen, Hoffman LaRoche, Genentech, Incyte Pharmaceuticals, Johnson & Johnson, and others. But with fewer potential lead compounds, the high price tag associated with the development of a drug, and the long lead time from the laboratory bench to the successful clinical trials before a drug product can become eventually approved by regulators like the FDA for use in the diagnosis, cure, mitigation, treatment, or prevention of diseases, R&D in the drug industry might also soon become a diminishing enterprise.
So this is the employment climate in which we are training our students nowadays. Industry no longer needs as many chemists as it used to. Aside from the lower demand, apparently, our students are not receiving the training that fits the requirements of potential industrial employers. Yes, we are still training our students the way we did some 40 or 50 years ago! Other fields, e.g., medicine, business administration, law, investment banking, etc., have also become more lucrative professions these days. So a career in chemistry is no longer as attractive to young people as it used to be.
In fact, chemical education is now at a crossroad and needs to be fixed if chemistry as we know it is to survive as a vibrant intellectual discipline. Like mathematics and physics, chemistry is one of the three fundamental disciplines in the physical sciences. But despite the renaissance in chemical science the past century, the chemistry curriculum for undergraduates has not changed much over the past 50 years or more. We still teach our courses more or less the way we did during the 1960s. To be sure, we use different textbooks to teach freshmen chemistry, organic chemistry, and physical chemistry, but the materials covered are largely unchanged. Chemistry departments are still largely compartmentalized into analytical, organic, inorganic, and physical chemistry divisions, but these subdivisions have become artificial, if not archaic. Research in chemistry is no longer divided by these boundaries. In fact, research advances in one area of chemistry increasingly depend on the concepts, methods, and tools developed by scientists working in another sub-discipline. For this reason, research collaborations have now become the rule rather than the exception. Yet, in the teaching of chemistry to our students, there has been little attempt made to consolidate the materials from the courses offered in the various disciplines into more "integrated" courses despite the increasing overlap in course contents among these courses. Instead the tendency has been to duplicate concepts and facts taught in another sub-discipline into an existing course. To treat more modern developments, we introduce additional advanced courses into the curriculum and hope that our students can pack them into their four-year course of study. In recent years, some chemistry departments have also added new sub-disciplines, such as chemical biology and materials chemistry, to the undergraduate curriculum, creating even greater barriers for curriculum reform. Inasmuch as piecemeal patching of the curriculum to circumvent the task of overhauling the chemistry undergraduate curriculum has finally reached diminishing returns, the time has finally come for us to meet this issue head on. The system is "broken" if not outdated, and we need to fix it.
Those of us who have had the good fortune to live through the past 50-60 years will tell you that we have witnessed a period of phenomenal growth in molecular science during the past century. By any measure, this has been an amazing century in the development of the science of chemistry. Clearly, we have collectively moved our science forward extremely well. In fact, we have been so successful that some chemists feel that the science of chemistry has matured. Certainly, it is possible that fundamental chemistry has matured, as we now know all the principles that will allow us to make predictions about the structure and properties of molecules, including their reactivity. In recent years, it has also become evident that chemistry is entering an applied phase, just as the sciences of thermodynamics and fluid mechanics have done so some 50 years ago.
For a number of years now, chemistry has been feeding into a number of other disciplines, including the life sciences, materials sciences, atmospheric sciences, earth sciences and environmental sciences, and even the engineering sciences. As expected, as these fields have become "molecularized", they have developed rapidly. For example, witness the recent rapid development of the life sciences, following the introduction of molecular biology, structural biology, and biochemistry/biophysics into biology. The same can be said about materials sciences. These are the areas where there are problems to be solved and where the challenges are. In any case, these developments illustrate the power of the molecular approach in problem solving, and suggest that chemistry has emerged as the central science that feeds into many fields. For some years now, many chemists have been moving into these fringe fields to seek problems for their own research. The amount of research funding earmarked by government funding agencies as well as private foundations for these endeavors have increased dramatically in recent years as well. For those of us who are chemical educators, it means, of course, more service teaching, as we take over the responsibility of educating a new generation of "chemistry users" or "non-chemists" in our science, in order that these young people could solve problems in the peripheral fields they have opted for their careers.
"Is basic chemistry "dead"? Of course, not! Let us address the issue head on by considering the current status of chemical synthesis, probably the most important branch of fundamental chemistry. Even today, synthetic chemistry remains one of frontiers of chemistry. It is a cutting-edge field because we would never know what the synthetic chemist is going to make. This discussion is not about the "run-of-the-mill" molecule, but those new molecules that are merely mental conceptions until they have been made. Yes, chemists make molecules. If chemists do not design and build new molecules, who will? If synthetic chemists do not continue to develop new synthetic methodologies and make new molecules, there will be no new compounds for all of us to study or use.
Most importantly, without synthetic chemistry, there would
be no outlets for the synthetic chemist to channel his or her
creative energies, and there would be no new molecules to capture
our fancy and imagination. Indeed, synthetic chemistry remains
the most creative branch of the molecular sciences, and we cannot
conceive chemistry as a viable intellectual discipline without
chemical synthesis. Building things, whether these things are
molecules, or lego toy pieces, allows us to transform our mental
conception of three-dimensional images into real objects, and
even our molecular fantasies into reality. As basic scientists,
we all dream, we must dream, and for many chemists, chemical synthesis
enables us to fulfill that dream! So long as there is space in
the field to dream, chemistry remains a fundamental science.
There will be those among us who will tell us that synthetic chemistry
has also matured. Indeed, the field has advanced over the years
to the point that a synthetic chemist can make almost any molecule,
provided that the molecule does not violate the principles of
chemical bonding as we understand these principles today. To address
this issue, we must ask ourselves whether or not the science of
chemistry has progressed to the point whether we have all the
rules of chemical bonding totally in hand. Do the quantum chemists
have any more surprises up their sleeves? Or have they pretty
much exhausted the realm of possibilities? If history is any judge,
there will be more surprises, and again, we can expect the surprises
to come from those who do not know any better than to mix things
together, namely, the synthetic chemist. Some of us are old enough
to remember the noble gas fluoro- and oxo- compounds (Figure
5A) that were first prepared during the 60's, before it was
evident that noble gas compounds were possible [6].When
buckeyballs or fullerenes (Figure
5B) were first reported [7],
some chemists were initially skeptical that a new form of carbon
had indeed been discovered. The moral of the lesson is that chemistry
is still an experimental science, and chemists must continue their
search for new molecules and try to synthesize them. This is the
kind of basic science that chemists should never give up.
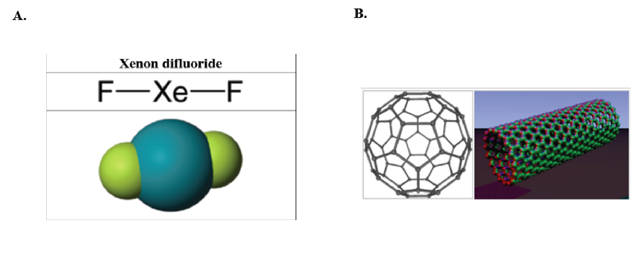
Figure 5. (A) The simplest noble gas compound: xenon difluoride (XeF2). (B) Buckminsterfullerene C60 (left) and carbon nanotubes (right) are two examples of structures in the fullerene family.
Here is an example of how we can learn from nature to come up with new chemistry to carry out difficult chemical transformations. One of my research interests during the past 20 years has been to understand how nature converts methane efficiently into methanol in methanotrophic bacteria at room temperature. This chemistry is extremely difficult in the laboratory because the C-H bond is very inert (the C-H bond energy is very high, 105 kcal/mole). For this reason, the search for a catalyst or a process that can convert methane to methanol under mild conditions has been one of the holy grails of organic chemistry(Figure 6). My laboratory has spent almost 20 years trying to understand how nature carries out this difficult chemistry. A membrane-bound enzyme called the particulate methane monooxygenase (Figure 7) can carry out the conversion of methane into methanol with high efficiency [8], with a turnover frequency approaching ~1 methane molecule per second per enzyme molecule. It turns out nature has invented new chemistry based on a unique tricopper cluster [8] to perform this new chemistry. Taking advantage of this new chemistry, my laboratory has invented a new catalyst (Figure 8) that is capable of efficient conversion of methane to methanol, but also all components of natural gases, namely, methane, ethane, propane, and butane to their corresponding alcohols at room temperature (Figure 9)[9,10]. The moral of the story is that there is still chemistry that we haven't anticipated based on what we know, and even first principles that we can exploit for chemical synthesis, like the controlled oxidation of a small molecule like CH4. There are other examples, like the reduction of CO2 to form CH4, the fixation of N2 to form ammonia, and the photo-oxidation of H2O to O2, where we need to develop new chemistry to accomplish these chemical transformations efficiently under ambient conditions of temperature and pressure. But the development of these new methods more likely will involve understanding how nature carries out these processes in the biological system and then designing new catalysts to accomplish them. This is a new approach to chemical synthesis, called "Integrated research for chemical synthesis", which involves learning from nature to come up with new chemical principles for chemical synthesis. Another successful story is the synthesis of natural products from peptides that are genetically coded and expressed on the ribosome of a cell and subsequently transformed into rigidified frameworks by post-translational modification [11,12]. Yes, there is still intellectual content in the science of synthetic chemistry.
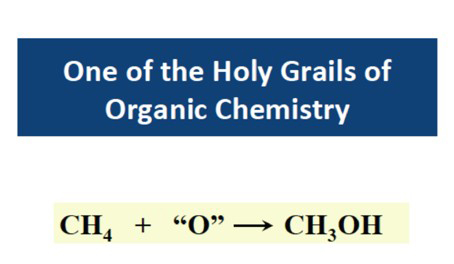
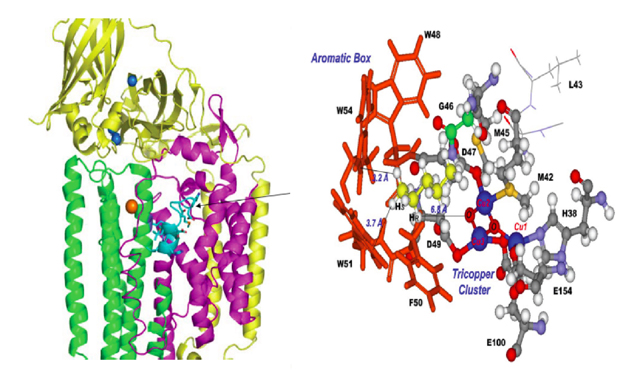
Figure 7. Structure of the particulate methane monooxygenase protein isolated from the methanotrophic bacteria Methylococcus capsulatus (Bath), an enzyme capable of efficient conversion of methane into methanol at room temperature. Left: Ribbon diagram of the polypeptide backbones associated with the three subunits: PmoA (magenta); PmoB (yellow); PmoC (green). Reproduced from [8]. Right: The active site of the enzyme comprising the catalytic tricopper cluster (copper ions in blue) and the hydrocarbon binding pocket ("aromatic box") sequestering a n-pentane molecule (yellow), the largest hydrocarbon substrate that can be oxidized by the enzyme. Reproduced from [10].
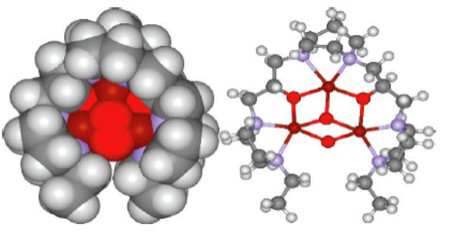
Figure 8. A tricopper complex that mediates efficient conversion of methane into methanol at room temperature and normal pressures. Atoms: copper (·); Oxygen (·); nitrogen (·); carbon (·); and hydrogen (·). Reproduced from [9].
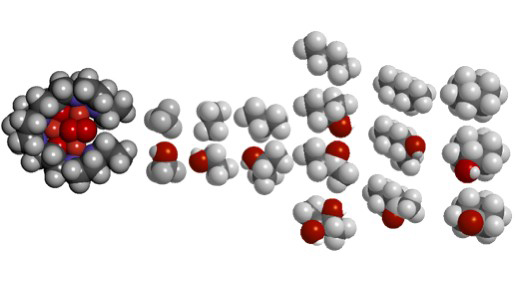
Figure 9. A view of the tricopper complex (left) is shown depicting the molecular surface of the pocket recognized by the hydrophobic alkane during binding and subsequent catalysis. Several small alkanes that are oxidized upon activation of the tricopper cluster are also shown, together with their oxygen (·)-containing products. From left to right: methane is oxidized to give methanol; ethane to ethanol; propane to 2-propanol; n-butane to 2-butanol, 2,3-butanediol, and 2-butanone; n-pentane to 2-pentanol and 2-pentanone; and cyclohexane to cyclohexanol and cyclohexanone.
Fewer fundamentally new reactions are being discovered these days. Most synthetic chemists apply existing methods toward the building of new target molecules, or perform molecular engineering. However, there are still intellectual frontiers out there for the synthetic chemist to explore, e.g., the rational design of catalysts to mediate various types of synthesis, like asymmetric synthesis and organo-catalysis; the design of molecular machines to transmit chemical signals from one part of a molecule to another, to link chemical reactions occurring in different regions of space for the inter-conversion of different forms of energy, for coupled directional transport of molecules, etc.; the development of methods to allow chemical syntheses and chemical processes to take place in H2O or in environmentally friendly solvents, and with economy of atoms, energy, and without waste production, the so-called green chemistry; and so on. Progress in some of these areas of chemistry requires the design and synthesis of large molecules.
The chemistry of large molecules is definitely an intellectual frontier. In this area, there is considerable amount of room for intellectual thought, the development of concepts, and the definition of new research problems. Here, there are exciting opportunities for the synthetic chemist to build new molecules with novel properties that other chemists can study and exploit further. We all know about big molecules like DNA, RNA, proteins, and how these molecules can be used to store information, transfer information, transcribe information, control the flow of information, encode and build the molecular machines to mediate the synthesis of molecules, store energy of various forms, and control the conversion of energy from one form to another. As chemists, we should be able to design and assemble new macromolecular systems that can perform many of the same kind of functions as well as different ones. The opportunities here are tantalizing and exciting. To synthesis some of these molecules efficiently, however, we need to establish the intellectual framework and come up with new design principles.
Why are large molecules fundamentally interesting and significant? Large molecules have many energy states associated with the relative motions of the atoms in the molecule. The number of energy states with roughly the same energy is often referred to as the density of states at that energy value. In principle, by controlling the location of the hard (covalent) and soft (hydrogen bonds, salt bridges, van der Waals, hydrophobic) bonds throughout the molecule, and the relative strengths of these interactions, one can influence the density of states and the ensemble of energy states that are thermally accessible. The dynamical properties of the folded structure not only determine the distribution of energy states, including the states that are thermally accessible at a given temperature, but also the kinetic barriers associated with the inter-conversion among these energy states. These kinetic barriers are of particular relevance, because they determine the flow of energy and information from one part of the three-dimensional fold to another when an impulse is delivered to the molecule. If properly designed, such a system can do vectorial chemistry by exploiting the conformational transitions that occur in the molecule according to a kinetically controlled sequence. Thus, the chemist can begin to think about assembling a molecular machine that can perform chemical work.
Lest there be any misunderstanding, there is no reason why
new chemistry cannot be discovered in the studies of materials,
on biological systems, or even in the earth sciences. As noted
earlier, nature has had 6 billion years of evolution to come up
with new chemistry in the biological system, some of which probably
has yet to be uncovered by us. Under the extreme pressures within
the earth's mantle, it is possible that there are new chemistry
and chemical transformations that we do not know about. These
are the kinds of fundamental discoveries that the modern chemist
should be excited about.
Finally, there is certainly room for applied chemistry in the
setting of a chemistry department. However, the intellectual content
of the science should be high and the work should be performed
with the highest intellectual rigor.
Now that I have assured you that the intellectual opportunities are rich and the future beckons some of our best young minds to work in these frontier areas of chemistry, let us now turn to the practical issue of how we as chemistry educators can inspire and motivate some of these young people, and nurture them so that they can eventually develop into the future Linus Pauling or Robert Woodward, two of the greatest chemists who lived during the past century. Our goal as instructors in the setting of a college or university is to develop and impart knowledge, mentor students, motivate, inspire and even challenge them. Toward this end, we must help them generate skills in critical analysis, involve them in meaningful intellectual inquiries and discourses, and to promote their accomplishments. This is a tall order, which many of us are not trained or prepared to meet.
Since the 1980s, there has been an alarming drop off in the number of young people selecting chemistry as their field of study in the colleges and universities, or choosing teaching and research in chemistry as the career path. Even those students who pick chemistry as their majors are disenchanted by the way chemistry is presented to them in the classroom, and become turned off by the lack of intellectual excitement of our science that they ultimately turn their attention to other fields for graduate study. The problem is worldwide. It is happening in the US, France, Germany, even Asia. Now our upper division courses are taken by only a handful of students with motivation toward a career in chemistry, and even these students might not end up pursuing graduate study in chemistry. To accentuate the problem, the average quality of the student pool has also changed substantially since the mid-1980s. Generally speaking, we have been less successful in recruiting the top students to pursue chemistry or engaging the best young minds to join us in our work. The top young people do not wish to pursue basic chemistry these days. They prefer the biological sciences, if they are interested in science; otherwise engineering, medicine, economics and business management. In the long run, chemistry departments will have difficulty finding exciting young people to fill the positions we are vacating when we retire, if we allow this situation to persist for much longer than a generation.
We must all assume part of the responsibility for this demise. Just take our freshman chemistry course as an example. We are still teaching the course the same way it was taught in the 60's, when some of us were taking it as a student, even though some of the material our professors used to teach us has already trickled down to the high school chemistry course. Instead of using the opportunity to excite the students with the achievements academic as well as industrial chemists have made during the past 50 years and to give them a sense of the directions in which modern chemistry is heading and where the discoveries are likely to be made, we teach more or less the general chemistry course. No wonder we turn them off, and even kill off their motivation toward our science. This having said, however, I must confess that few of us are prepared to teach the new introductory chemistry course referred to above. So it is time for a few of us to rewrite the textbooks for the general chemistry course.
New textbooks should be written to reflect all the discoveries and progress made in our science the past 50 years, especially with greater emphasis placed on structuring the material around fundamental principles over the presentation of factual information. These texts should be less encyclopedic and the presentation more pedagogical. We fail as educators if we merely feed information to the students, and do not take the opportunity in the classroom to draw connections between tidbits of information from here and there, and from different fields. Our students need more insights, not facts.
We also need to promote changes in the teaching of science in our system of secondary and tertiary education in order to jumpstart the development of our science toward the next exciting age. Because of the information explosion during the past century, we, as high school teachers and college professors, have been packing more and more information into our courses. During the formative years, students should learn the chemistry that relates to day-to-day experiences. A high school course in chemistry should emphasize elementary chemical principles, including the principles of chemical bonding, states of matter, and the fact that modern chemistry is structurally based. While these principles should be eventually taught in greater depth in different courses as part of the chemistry undergraduate curriculum, the introductory chemistry course should emphasize discovering new chemistry, the excitement of discovery, in addition to the chemistry that relates to the applications in the applied disciplines, including materials chemistry, life sciences, environmental chemistry.
During the past 50 years, courses in chemistry have been more and more specialized. As our courses have become more specialized, our students are finding it more and more difficult to identify that common intellectual thread that presumably must unite the body of knowledge that we are passing on to them. "Fire hosing", as this form of classroom teaching has become to be known leads to information indigestion on the part of the students. We must teach the students how to sample, just as we are supposed to do with the various dishes in a ten-course Chinese banquet so that we can enjoy the feast. If we over-indulge and suffer from indigestion, the meal becomes less of a pleasurable experience. The same obtains with fire hosing in the classroom. Here we risk choking off the students' scientific motivation. Sometimes, we even kill off the students' intellectual curiosity and stifle their creative energies, contributing to their anti-intellectual behavior.
In my judgment, by simply passing on what we know, we fail to educate our young people in the two most important skills that will serve them best the rest of their productive adult lives: (1) the ability to think, analyze, innovate, and create; and (2) the intellectual curiosity to continue to explore, learn, and dream. To be sure, our intentions are good. However, by being over-zealous in passing on everything we know to our students, we fail to meet our obligations as teachers and mentors.
In order to solve the important chemical problems of the future, whether these problems involve macromolecular systems, advanced materials, and even the complex problems related to our environment, we need scientists with diverse backgrounds and training. Accordingly, we need to train our students to think more broadly and to learn to do research across the entire discipline, and even inter-disciplinarily. At the very least, we must train our young people to work with other scientists who are trained in fields other than their own, and we must educate our students to develop the skills to communicate with other scientists. Unfortunately, as Asians, most of us prefer to work by ourselves, rather than as teams, because of our cultural upbringing. This cultural trait, I am afraid, is detrimental to the science of the future and we must slowly rectify this shortcoming through education at an early age.
We also need to streamline our undergraduate curriculum to include more courses that are less discipline-driven so that young people could learn to relate the ideas and concepts from a variety of fields. Aside from a solid training in basic mathematics and physics, chemistry undergraduates should be given the opportunity to do more intellectual sampling in related molecular sciences, e.g., biology, materials sciences, and the environmental sciences. Chemistry courses should also be less specialized and more principle-driven, focus on more modern issues, and include opportunities to allow the students to develop not only their analytical skills, but also their ability to synthesize ideas and to define and formulate scientific problems. The teaching of chemical synthesis should be integrated to include organic, inorganic, and organometallic systems and approaches. A course in physical chemistry should place more emphasis on condensed matter and include the treatment of biological and other complex systems. Of course, in order to accomplish these curriculum changes, we as teachers will need to exercise some self-discipline in the selection of topics to present in the classroom. It will also help if we can get together to improve the coordination of the presentation of the matter in various courses and to eliminate some of the excessive duplication that currently exists among them.
As a discovery science, chemistry remains an experimental discipline, so an integrated laboratory/research proficiency that provides hands-on experience as well as opportunities for exploratory activities should go hand-in-hand. Although nowadays we try to understand chemistry mostly in molecular terms, chemical reactions are still being carried out with liquids, solids, solutions, so students need to understand the collective behavior of molecules and their properties. In this context, I must also include the computational laboratory, where state-of-the-art software packages are presently available for quantum chemistry, molecular dynamics, predictions of molecular structure, molecular simulations of surfaces and solids, supramolecular structures, mesoporous materials and nanoparticles, catalysts, statistical physics, and even chemical reactions. With the advent of modern computing technology, parallel processors, and molecular graphics, molecular simulation are becoming an increasingly important tool in modern chemistry. With a generation of younger students highly proficient with information technologies, new approaches in chemical education should be developed to take advantage of the power of modern information technologies and modern teaching methods, including the promotion of self-learning via accessing the colossal and diverse information sources available from the World Wide Web, e-learning, group learning via communication on social media and professional networks, and even the incorporation of online technology into the curriculum. These forms of learning are clearly very efficient, though I am not convinced that they are conducive to learning concepts in depth. The approach is less structured, but so is the process of self-learning as we go through life.
In any case, there is clearly plenty of room for innovation
in chemistry. There is no single silver bullet that will work
for all scenarios. Every institution or department must come up
with its own formula to satisfy local circumstances. To illustrate
the power of flexibility, I cite how one university in the US
has been making important strides toward the teaching of introductory
chemistry to its freshman class. The University of Virginia at
Charlottesville has been doing a very interesting experiment for
many years. Students intending to major in chemistry or related
disciplines no longer take the freshman course. They move into
organic chemistry or physical chemistry right away. The freshman
course is still offered. In fact, there are two freshman courses,
one catered to the life scientists and premed students, and the
other to engineers, materials scientists, and physicists. The
course material is tailored to the interests of the two groups
of students. Students are free to move from track to track as
their interest change. They merely start at a place that they
could handle. The outcome has been interesting. The rate of retention
of the chemistry majors has become much higher. More students
develop interest in chemistry as a science and move on to the
chemistry major track and eventually go on to graduate school
in chemistry or the biological sciences.
Finally, as chemical educators, we train chemists not only for
industry, academia, but a multitude of other professions. We need
to develop chemists with broad chemical knowledge and perspectives
for meaningful, productive and rewarding careers as secondary
school science teachers, in the making of public policy, risk
analysis, chemical safety, environment protection, environmental
law, patent law, and so on. Our concern should also extend beyond
chemistry in the college curriculum to the role of chemistry in
K-12 education. Making young people to become more literate about
chemistry should be one of our goals as well. As the central science,
chemistry should be just as important as reading, writing, mathematics,
and languages in our education.
As a chemist who has taught chemistry in a major research university in the US for almost 50 years, I am sharing with you my own impressions of how chemical research and undergraduate teaching in chemistry have evolved in North America during this period. These views are totally based on my own observations and experiences as part of my teaching and research, tempered by my interactions with undergraduates I taught, the research students I mentored, and the colleagues with whom I shared these responsibilities. Again, the points that I have try to articulate here are totally my own personal views and should not be construed to represent those of my colleagues, or even the chemistry community at large. It is possible that my experiences may not be the same as what you have experienced or are presently experiencing now here in Asia, and for that I apologize for having wasted your valuable time in reading this article.
However, as a chemist and an educator, I care about the development of chemistry as a science and a profession, and I care deeply about the personal, intellectual and professional development of prospective budding young chemists in the global village of which we are now all a part. It is for this reason that I am sharing my vision of the future of chemistry and chemical education with you. With the optimism that I have framed my remarks, I hope that I have inspired some of you to go forward to improve chemical education to the best we know how.
This article grew out of the opening lecture that I presented at the 5th International Conference of Network for Inter-Asian Chemistry Educators (5th NICE) held at the National Pingtung University of Education (NPTUE) in Pingtung, Taiwan during 25-27 July 2013. I thank the organizing committee for the invitation to deliver this lecture. I am also grateful to NPTUE for travel support that made it possible for me to participate in this conference.