Changhua 50058, Taiwan
*yangsp
Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-103/Received August 31, 2013._
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
This experiment developed at a microscale level was in response
to green chemistry for undergraduate laboratory instruction. Usually,
all equipments used for the synthesis of gold nanoparticles (AuNPs)
must be cleaned with aqua regia. These practices are harmful to
human health and detrimental to the environment. In our experiment,
one-pot reaction was used to directly synthesize colloidal Au
in a clean vial for a short time. The procedure was very easy
to proceed and had a high success rate. Characterization of colloidal
gold at microscale level was also straightforward, which included
three aspects: Tyndall effect, aggregation, and spectrum. A novel
characterization regarding the AuNPs aggregation is really valuable,
in which the aggregation was tested on a white paper coated with
a layer of wax using two liquid balls of colloidal gold. We found
that all students could successfully synthesize intense ruby red
color of gold nanoparticles in 10 minutes, and managed to characterize
the colloidal Au in 10 minutes in this microscale experiment.
The amount of HAuCl4 used was only one
tenth of those in typical synthesis. The spectrum results in lmax obtained by
students were in a range of 520-532 nm. Testing for the AuNPs
aggregation and the Tyndall effect were positive. This experiment
is consistent with five principles of green chemistry. Working
at this experiment has many advantages: reducing the time, cost
and waste, and encouraging the students to think about environmental
protection. The laboratory instruction revealed that the overwhelming
majority of the students had positive responses in the cognitive,
affective and psychomotor domains. Moreover, the students felt
more valuable that they have an opportunity to permanently preserve
their own products of AuNPs by stabilizing with PVP in the original
vial.
Keywords: microscale experiment, green chemistry, gold nanoparticle, and laboratory Instruction
Instructional Strategies and Considerations
Gold nanoparticles: Typically, gold nanoparticles (AuNPs) are synthesized in an aqueous solution by reduction of chloroauric acid (HAuCl4, hydrogen tetrachloroaurate). After dissolving HAuCl4 in a deionized water, the solution is rapidly stirred while a reducing agent, such as sodium citrate, is added. This results in Au3+ ions to be reduced to neutral gold atoms. As the gold atoms from gradually, they gold atoms accumulate to form AuNPs, and the solution becomes colloidal. Currently, several synthetic methods for AuNPs, especially in size-and-shape controlled, have been developed [1-3], and its widespread application has been reported [4-6].
Gold nanoparticles and macroscale experiments: With the congress in the emerging discipline, many chemical experiments focused on the synthesis and characterization of AuNPs for the laboratory instruction have been present in chemical education journals [7-9] and on the Internet [10-12]. However, all of these experiments were designed in a macroscle level, rather than in a microscle level. For example, an experimental procedure mentioned: "Add 20 mL of 1.0 mM HAuCl4 to a 50 mL Erlenmeyer flask Slowly add 2 mL of 1% Na3C6H5O7·2H2O to the 50 mL flask." [12] In typical procedures, all equipments used in the synthesis and characterization of AuNPs must be cleaned with aqua regia [13-15]. For instance, an experimental procedure described: "Nanoparticles are normally prepared using specially cleaned glassware and avoiding any exposure to organic materials. The Turkevich paper reported cleaning with aqua regia." [15] Unfortunately, these chemicals used in the experiments are harmful to human health and detrimental to the environment. Furthermore, in reviewing on the synthesis and characterization of AuNPs, we have not found any of the general chemistry laboratory textbooks [16-20] provided undergraduate students with such experiments as teaching materials.
Microscale chemistry: In the 1980s, the microscale chemical experiment (MCE) has begun developing in three agencies (Bowdoin College, Merrimack College, and Brown University) of the United States [21]. It started in organic chemistry experiments originally, and spread to the general chemistry, inorganic chemistry, analytical chemistry, and environmental chemistry experiments [21]. The MCE refers to the use of miniaturize instrument and equipment devices, and the reduction of reagent quantity to a range of 1/10-1/1000 of that in normal experiments. The MCE is a good way to achieve the concept of green chemistry. In the industry, green chemistry is mainly to reduce/eliminate the use and generation of hazardous substances in manufacturing process for preventing harm to the environment. In the education, green chemistry is involved in chemical laboratory instructions. The MCEs and green chemistry experiments can improve the quality for students' interest in learning and teachers' teaching. The benefits of implementing MCE and green chemistry laboratory include reduced reaction time, improved safety, and major cost savings [21]. Green chemistry and microscale chemistry are complementary pedagogies, allowing the ideas of resource reduction, material substitution, and exposure minimization to be brought effectively into the academic laboratory [21].
Implementation of microscale chemistry: The National Microscale Chemistry Center (NMC2) in the United States was established in January 1993 to promote the use of microscale chemistry as a means of eliminating toxic waste at the source. Its major initial focus is on the offering of workshops, seminars and publications on the operation and advantages of conversion of laboratories to a microscale level [22]. Microscale Chemistry is performed by means of drastically reduced amounts of chemicals, safe and easy manipulative techniques, and miniature labware and high quality skills [22]. Journal of Chemical Education offers the Microscale Laboratory column. Since 2005, this Journal has published about 50 MCEs, which were mostly organic chemistry experiments [23], and fewer experiments in general chemistry [24]. Our experiment focuses on the development of the synthesis and characterization of AuNPs at a microscale level.
Green chemistry: The twelve Principles of Green Chemistry were originally published by current assistant administrator Paul Anastas of United States Environmental Protection Agency and John Warner [25]. The twelve principles are: prevention, atom economy, less hazardous chemical syntheses, designing safer chemicals, safer solvents and auxiliaries, design for energy efficiency, use of renewable feedstocks, reduce derivatives, catalysis, design for degradation, real-time analysis for pollution prevention, and inherently safer chemistry for accident prevention [26-27]. Our experiment developed at a microscale level attempted to respond to green chemistry for undergraduate laboratory instruction.
Chemistry-major freshmen students as individuals, a group of one student, worked this AuNPs experiment at a microscale level for a three-hour laboratory sessions. The students were first introduced to experimental process, content background, especially in the synthesis and characterization of AuNPs, and concepts of microscale chemistry and green chemistry. The students then manipulated experimental procedures and wrote their own reports and learning feedback. By the way, the synthesis of AuNPs (excluding the characterization) at a microscale level had been performed within 40 minutes in an event that a class of high-school students visited the chemistry of department in our university. All of the students were a first experience in the synthesis of AuNPs. Valuably, they can success to synthesize colloidal AuNPs and most of the students had positive responses for the synthesis. Examples of the students' feedback are as follows: "I am so happy that I am able to synthesize gold nanoparticles myself, and bring it to home permanent preservation." and "I like this experiment, gold nanoparticles is very easily synthesized, and the change in color is attractive."
The main pedagogical characteristics of this laboratory instruction included two aspects to be expected. In terms of pedagogical characteristics: emphasis on students' reflections in between microscale and macroscale, accent on students' introspection in green chemistry and environmental protection, as well as emphasis on the significance of multi-style design with chemical experiments. In terms of content knowledge and laboratory technique: comprehending the synthesis and characterization of AuNPs, proficiency in the technique of microscale chemistry, expertizing in taking UV-visible absorption spectrum and in testing for the aggregation using liquid balls, as well as understanding in nanoparticles and green chemistry.
Many teachers/instructors misunderstood that this experiment is very expensive due to the use of gold. Actually, each experiment/student/group only spent in a low cost experiment at microscale level. The price of hydrogen tetrachloroaurate(III) tetrahydrate (HAuCl4·4H2O) is about USD 100 per 1.0 gram. A 0.041 g of HAuCl4·4H2O can suffice for 50 experiments, or for 50 students/groups. By calculation, each experiment/student/group merely spent (USD 100 / 1.0 g) x (0.041 g / 50 students) = USD 0.082 / student. That is, it is equivalent to JPY 8.1/student, TWD 2.5/student, and GBP 0.052 / student. Thus, the use of gold for this microscale experiment is cost-effective.
Chemicals and Equipments
The following chemicals and equipments are needed for each
student/group to perform this microscale experiment, in addition
to a 100.0-mL volumetric flask, three 100-mL Erlenmeyer flasks
equipped with silicone rubber stoppers and two spectrophotometers,
which are intended for a whole class.
Preparation of solutions
The instructor/TA needs to prepare the followings:
Experimental Procedures
Synthesis of gold nanoparticles (AuNPs): With a clean Pasteur pipette, add 40 drops (2.0 mL) of 1.0 mM HAuCl4 and 10 drops (0.50 mL) of 34.0 mM Na3C6H5O7 into a 3-mL vial which is not need to clean and directly to use. Close the cap tightly. Place the sealed vial to the a half full of boiling water in a small beaker. Heat the solution until it shows a deep red color (about 10 min). Remove the vial from the beaker. Allow it to cool.
Characterization of AuNPs: In taking the Tyndall effect: the remaining solution in original vial was feasible using a laser pointer. In aggregation and stabilization, placing only 2 x 2 drops of AuNPs solution on quarter sheets of white paper coated with a layer of wax to form two liquid balls are sufficient in the comparison of color change by adding a few granules of sodium chloride to a ball. In taking the spectrum, pouring only 15 drops of AuNPs solution into a 1.5-mL disposable plastic cuvette and two-fold dilution was practicable.
Stabilization of AuNPs: To preserve AuNPs under stable conditions, put the diluted solution in the cuvette into the original vial. Put two drops of 0.3% PVP into it. Cover the cap tightly and shake the mixture uniformly. You can permanently preserve in the vial the AuNPs solution you synthesized.
Safety Precautions
Chemical splash goggles, protective clothing and gloves must be worn throughout the entire experiment. The hot water or boiling water baths should be handled with care to avoid burns. Chemicals used as much diluted solutions, 1.0 mM HAuCl4, 38.8 mM Na3C6H5O7 and 0.3% PVP, are not dangerous. However, hydrogen tetrachloroaurate(III) and sodium citrate are slightly hazardous and may cause borns/irritation of skin and eyes burns/irritants. Poly(vinylpyrrolidone) is hygroscopic and may cause eye, skin, and respiratory tract irritation. Hydrogen tetrachloroaurate(III), sodium citrate, and poly(vinylpyrrolidone) spilled on skin or your clothing should be washed off with water immediately and thoroughly. The three chemicals spilled above on eyes must be washed immediately, and inner surface of the eyelid must be rinsed with copious amounts of water for at least 15 minutes. Get medical aid immediately.
Microscale Synthesis
In terms of the microscale synthesis, the_mixture color was observed to change from colorless to_blue_to_purple to intense ruby red, indicates the regeneration of Au nanoparticles (AuNPs) as shown sequentially in Figures 1a-1d. The change colors are consistent with that in the macroscale synthesis.
Tyndall effect
In terms of the Tyndall effect, students can observe a beam of the red light through the AuNPs solution by irradiating using a laser pointer, whereas no beam is appeared in the HAuCl4 solution. Figure 2 shows that in left vial the AuNPs solution can be confirmed its colloidal presence and in right vial the HAuCl4 solution can be confirmed its true-solution property.
The particles in colloidal solution are large enough to reflect or scatter light in all directions. The Tyndall effect is due to light scattering when a light beam is irradiated to present through a colloid containing particles within 1-200 nm. For a light scattering, the colloid particles are stable and do not separate, while the suspension particles do separate on standing. In a true solution the dispersed particles are too small to scatter visible light. Based on the scatter light properties, intense ruby red color of AuNPs presented a stable and less separately scattering light means the size of these particles within 1-200 nm.
Particle Aggregation
In terms of the particle aggregation, students can clearly observe the change of color of the AuNPs solution from intense ruby red to dark gray by adding granular sodium chloride, as shown in Figure 3. This is because of increasing AuNPs size by adding electrolytes yields a blue-green color or dark gray shift from a ruby red color.
Electrolytes, such as sodium chloride, contain two types of ions, one is cation with positive charge and the other is anion with negative charge. Each Au nanoparticle has negative electrical charges; in this case citrate ions are on its surface, which allows the individual AuNPs to repel each other. As sodium chloride is added to the AuNPs solution, the Na+ ions are attracted to the negative charges of AuNPs so that this reduces the nanoparticles repelling each other. Thus they are able to aggregate, making larger nanoparticles. The size-color effect is consistent with the aggregation.
Absorption Spectrum
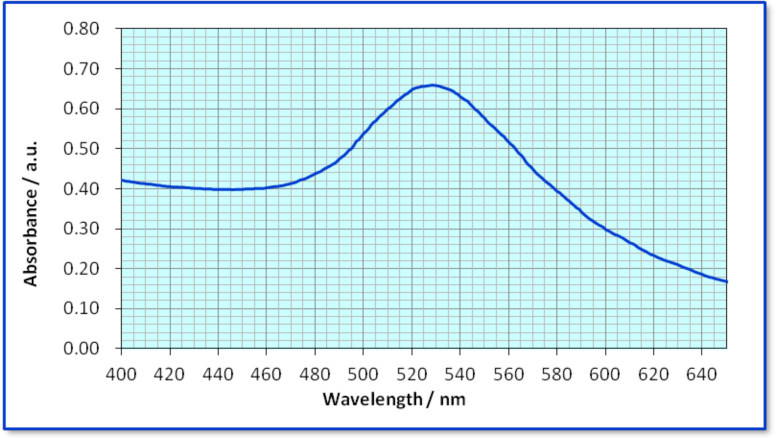
In terms of taking the spectrum, the results, in maxmum absorption wavelength (lmax) of AuNPs, obtained by students were in a range of 520-532 nm. The results are close to literature values, 520-528 nm. Figure 4 shows a student's absorption spectrum of AuNPs using a UV-visible spectrophotometer, and the lmax was 529 nm.
Learning Feedback
Students' learning feedback is summarized into three categories: cognitive, affective, psychomotor domains, and evaluated by the qualitative analysis - positive response, negative response, and combination of positive and negative responses. Representatives of students' learning feedbacks are described below.
- Cognitive domains (positive response)
"Waxing should be coated evenly on the paper to prevent
liquid balls (droplets) from penetrating into the paper."
"Electrolyte was added so that the negative charge of
gold nanoparticles on outter layer is offset away. This eliminate
the nanoparticles repel each other and produce aggregation."
"This experiment uses a very little amount of chemicals.
It is consistent with the idea of green chemistry" "Heating
a mixture inside a vial in a hot water bath makes the temperature
constant." "The microscale experiment in the
synthesis and characterization is really simpler than the typical
experiment. It is very easy to follow and understand in this way."
- Affective domain (positive response)
"This way at a microscale level eliminated the steps of
cleaning and rinsing equipments so that I improved operational
efficiency and reduced chances of failure." "The
microscale method eliminates troubles of cleaning equipments,
and reduces the difficulty of the operation. I believe that high-school
students can easily synthesize, identify and understand gold nanoparticles."
"I thought it was very joyful to preserve our own experimental
products the Au nanoparticles we synthesized ."
- Psychomotor domain (positive response)
"I followed the experimental procedure and found that
gold nanoparticles can be actually synthesized and characterized
in a more simple simpler way."
- Cognitive domains (combination of positive and negative responses)
"Coating a thin layer of white wax on a white paper to
make liquid balls is a good idea. Perhaps, coating wax inside
a petri dish is better because it can be cleaned and reused and
to be more environmental friendly."
We found that all students can successfully synthesize intense
ruby red color of the gold nanoparticles in 10 minutes, and facilitate
the characterization of the colloidal gold in 10 minutes in this
experiment at a microscale level which the amount of HAuCl4 used was only one tenth of those in a typical
synthesis at a macroscale level.
This experiment at a microscale level is consistent with five
of twelve principles of green chemistry: prevention, less hazardous
chemical syntheses, designing safer chemicals, reduceing derivatives
and inherently safer chemistry for accident prevention. In additional,
working in this experiment has many advantages: reducing the time,
cost and waste, as well as encouraging the students to think about
environmental protection.
According to the qualitative analysis of students' learning feedback,
the laboratory instruction using in our experiments reveal that
the overwhelming majority of the students had positive responses
in the cognitive domain, affective domain and psychomotor domain.
Moreover, the students felt more valuable that they have an opportunity
to permanently preserve their own results of Au nanoparticles
by stabilizing with PVP in the original vial.
The author thanks the National Science Council of Taiwan for financially supporting this research by grant NSC, and Dr. Jenn-Huei Lii for helpful suggestions and Yu-Wu Hsu for enthusiastic assistance. The author also thanks International Advisory Committee of 5th NICE (Network for Inter-Asian Chemistry Educators) Conference for the IUPAC Poster Prize awarded to the poster of this article.