E-mail: su-87168
Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-104/Received August 29, 2013.
URL =http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
This research deals with strategic applications of dynamic reaction
figures (DRF) in chemistry for problem-solving skills of students.
It focuses on reaction equations in the unit of redox chemistry
with ions and charge trans missions to clarify college-students'
misconceptions in chemical learning. This experimental study takes
sample models from two student groups, the experimental group
and the controlling group, with the total number of 95 college
students. All quasi-experimental approach and statistical analyses
for students' skills in problem-solving are summarized as follows:
(1) The experimental group students had better posttest achievements
than those of the controlling group students because they got
many skills in the unit of redox chemistry learning. (2) In the
comparison between their posttest and pretest results, the same
experimental group students had more significant and increasing
posttest achievements than those of controlling group students.
(3) In regard to different dispositions of students in chemistry,
experimental group students revealed more significant satisfactory
in learning of redox chemistry.
On the whole, students with applications of DRF showed more problem-solving
skills which could be applicable for their learning performances
of chemistry. °°
Keywords: dynamic reaction figures, problem-solving
skills, redox chemistry, quasi-experimental approach
Research approach and procedure
Brief introduction of chemistry learning
Instruments of applied methods
Orientation of achievement tests
Developments of satisfactory of students in chemistry learning
In order to promote students' learning performances, science
educators like Nakhleh and Malina [1]
proposed that college students at all levels should endeavor their
best for strategic chemistry learning, and they often felt much
dispirited in abstract chemical conceptions. The dynamic reasons
may be attributed to the fact that many students failed to get
control of chemical conceptions at the start of their learning
[2]. For the majority of college
students, to raise their scientific learning efficacy and techniques
was a difficult task, because they only picked out recited methods
for passing exams [3].
To have techniques of algorithmic operations paved the traditional
way for college students to decipher or answer similar basic chemical
conceptual problems [4].Whereas the
time came for students to solve intermediate or advanced chemical
conceptual problems, such as Electric Chemistry [5],
Stoichiometry [6], Chemical Equilibrium
[7], and Stereochemistry [8],
they usually became blurry with too much abstract and elaborate
chemistry in their learning. Schultz [9]
upheld that cognitive validity should take more skills with chemistry
narrations progressively and use expressions of visual animations
for increasing their curiosities and dexterities in chemistry
learning. There were different teaching highlighted supported
by visual advantages of molecules to enhance students' learning
recognition, which some science educators adopted such as representations
of computer animations [10-12], tactics
of problem-solving maps [13], or
dynamic reaction figures (DRF) [14, 15].
Many scientific educators [16, 17]
indicated that conceptual maps had more learning efficiency for
students' skills in science problem-solving.
In this study, the teaching design of this textual study of redox
chemistry was following applications of Schultz's DRF approach
and constructivism theories of Driver and Oldham [18],
with problem-solving skills, so as to get more meaningful learning
and create more substantial chemical conceptions.
This research focuses on major applications of DRF implemented
by 2D animations highlighted with chemical conceptions of ions
and charge transmissions. Two fundamental prospects of college
students' chemistry learning would be conducted as follows:
(1) To design dynamic DRF redox chemistry for students' skills
in problem-solving learning achievements
(2) To construct effective applications of DRF redox chemistry
for students' skills in problem-solving and examine their satisfactory
questionnaire
DRF could be a strategic application of conceptual maps, which
was full of conformity, clarity, visualization, variety, insight
and expansibility, all these could help students organize, classify,
analyze, assess and deduce, and promote critical thinking, not
only as a kind of study tactics, but also as a useful technology
of teaching and learning achievements [9, 14]. These strategies could make problem-solving
skills available by drawing DRF in their brain-storming presentations,
and these gradual highlighted analyses would conduct learning
for ions and charge transmissions in reaction equations of redox
chemistry. All above chemistry problem-solving skills would clarify
students' conceptual difficulties in their classes. Four characteristics
of DRF technology tools would be listed as follows:
(1)To enhance students' feedbacks for discerning and thinking
analyses
(2)To substantiate students' performances of chemical reaction
equations, principles, laws, and theorems
(3)To require students to compare other presentations of problem-solving
skills
(4)To upgrade students' learning performances, so as to overcome
difficulties, and solve
encountering problems.
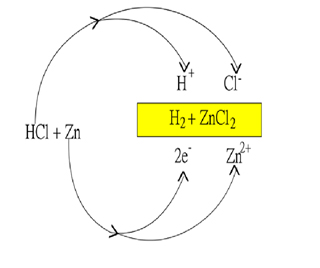
For a more effective DRF approach, Schultz [9]
developed the advanced chemistry learning, derived from the theory
of meaningful learning [19]. To take
the chemistry DRF example, we divided the experimental sample
into two hemispheres: the upper hemisphere to provide protons
(H+) can be called "Donor Hemisphere," and the lower
hemisphere to accept protons (H+) can be called "Accept Hemisphere,"
which is indicated in Schultz [9]
and Su [15]. Put two hemispheres
into a whole composition for the chemical reaction. To learn transmission
mechanism of ions or charges from the upper and lower hemispheres,
and to increase conceptual knowledge of chemical equations, we
need cartooned animations in demonstrating students' problem-solving
abilities in this study.
Up to recent years, problem-solving skills (PSS) in chemistry
researches have been one of major academic focuses [15],
and this study attempts to integrate many prevalent approaches
in special functions of PSS. Two chemistry questions of algorithmic
and conceptual PSS were treated critically as functional assessments
by Sanger and Phelps [20], Cracolice,
Deming, and Ehlert [21], and Domin
and Bodner [22]. Cracolice, Deming
and Ehlert [21] explored different
levels of reasoning conceptual skills to examine the gap between
conceptual questions and algorithmic questions of problem-solving
abilities. To train well-equipped scientific reasoning skills
would lower the above gap phenomena, and upgrade constructions
of students' conception. To integrate DRF into highlighted chemistry
learning would increase conceptual reasoning skills and construct
students' abilities in problem-solving.
In the modern scientific era of information technology, integrated
applications of highlighted DRF with creative scientific technologies
will become a dominant multi-functional approach from day to day.
To develop strategic applications of DRF technologies, this study
explored fundamental scientific knowledge [23,
24] in the unit of redox chemistry learning. Teaching tools
would be employed in designs of the experimental chemistry process
such as Flash Animations, combined with experimental apparatus,
and developments of multimedia technology including sounds and
graphic arts.
In order to have a detailed data discussions and analyses, 95 junior college freshmen were selected from the same class into two groups, the experimental group (47ps) taught by the integrated DRF technologies, and the control group (48ps) by lecture-based teaching methods without any assistance of DRF technological tools. The characteristics of the two different student groups who completed a 6 hour program in the three-week DRF animated schedules of chemistry during the 2012 academic year were discussed below.
To achieve effective teaching goals of redox chemistry, this
study proposes several presentations of macroscopic and microscopic
conception of dynamic changes with the integrated DRF technology
in chemistry to facilitate students' learning performance in the
following six research procedures:
(1) Set up the learning target goal
(2) Find out appropriate integrated DRF technology for learning
in redox chemistry
(3) Integrate DRF with animated demonstrations
(4) Stimulate learning activities with DRF teaching models
(5) Use quasi-experimental approach in the study project
(6) Assess students' performance in chemistry learning
Applications of computer technologies in chemistry; for example, integrated animations, static charts, and descriptions of learning performances were constructed as DRF modules. All chemistry teaching was divided into several relevant and meaningful DRF modules. The cognitive programs of redox chemistry designs, teaching methods, teaching situations, course technologies and satisfactory of students, were included in these major DRF modules. All these DRF modules were organically combined together to create new reliable programs and applications for students' skills in chemistry learning. Demonstrations with power points were presented in the experimental group of animated DRF modules. Figure 1 summarizes parts of Flash Animations; each animation would last for almost 20 seconds. In the experimental group, students had a 5 minute practice with animations during demonstration spans.
To achieve effective problem-solving skills, a quasi-experimental approach for questionnaire tests was used in this research, together with different criterion related to statistical analyses of students' learning efficiency and performances. This research design included pretesting, target-group teaching, posttests and questionnaire evaluation of satisfactory in chemistry learning. All research methods of pretests and posttests, experimental teaching, learning satisfactory questionnaire, and statistical analyses of achievements and satisfactory of students in chemistry learning, made students get involved in positive learning efficiency for promoting their problem-solving ability in chemistry.
Cognitive knowledge was incorporated into students' achievement tests in pretests and posttests. The original draft test was designed by educators [23, 24] and approved by four senior chemistry professors. To analyze the achievement tests, the reliability of Cronbach's _ coefficients was examined in statistical methods to determine the internal consistency of questionnaires. The _ coefficients obtained for redox chemistry in pretests and posttests were 0.76 and 0.75. DeVellis [25] regarded the 0.70 reliability as the minimum acceptable reliability. Both pretests and posttests were employed in the same method to record changes and detect differences students' achievements in chemistry learning.
The draft 30 test items were included in the questionnaire
for assessing satisfactory of students in chemistry learning.
On the whole these test items corresponded to the author's revisions
of the draft design [12, 15].
Likert-type scale was also employed in the questionnaire. For
constructing better content validity, this study asked two science
educators, two scientific philosophers and two educational psychologists
to perform advisors make revisions and examine the survey. To
increase the constructive validity, 296 copies of pretests were
taken into considerations for factor analyses. The first results
of factor analyses for the Kaiser-Meyer-Olkin (KMO) data (0.943)
and _2 data (5621.899) of Bartlett spherical investigation (the
degree of 300 freedoms) proved significantly important, so factor
analyses were deemed suitable. Three aspects were considered in
main component analyses of the questionnaire. The Eigenvalues
obtained were above 1.0 with an accumulative explanation variation
of 65.341%. These Eigenvalues of three aspects were 3.778 (9 items),
3.475 (5 items), and 3.183 (11 items). The Cronbach's _ value
could correspond to 0.930, 0.893, and 0.938 as shown by internal
consistency inspection of the Cronbach's _. There were
totally 25 items in the questionnaire (see Table
1) which could be classified into three dominant aspects:
Q1, Q2, and Q3.
Q1, to integrate DRF presentations for satisfactory of students
Q2, to investigate satisfactory of students in chemistry learning
Q3, to inspire active participations for students' satisfactory
in chemistry learning
Three dynamic aspects Q1, Q2, and Q3 were focused on students'
satisfactory feedback in learning from the factor analyses. Factor
loadings of all items were indicated in Table 1.
SPSS 12.0 Windows software was combined with some statistical analyses for all information before and after the experimental teaching on the questionnaire.
This study focused on constructing DRF modules based on educational principles of chemistry learning at a Taiwan technical college and exploring two student groups learning performances with specific implementation conditions and learners' performances of integrated DRF in chemistry.
|
|
|
|
|
|
|
1. Each unit of the DRF-integrated teaching program matches my need to study. |
|
|
| 2. I take part actively in related DRF learning. |
|
||
| 3. I have confidence in DRF-integrated courses which are helpful for my study. |
|
||
| 4. Integrated DRF can provide my necessary aids to study in every subject. |
|
||
| 5. The teaching style of DRF instructors is lively. |
|
||
| 6. The teaching method of DRF instructors is flexibility for students. |
|
||
| 7. The instructor of my DRF class cares my learning performances. |
|
||
| 8. The instructor of my DRF class often encourages me to study. |
|
||
| 9. I am satisfied with the teaching performances of my DRF class instructor. |
|
||
|
|
10. Our classmates can actively participate in the teaching activities during DRF class. |
|
|
| 11. Our classmates can take part in discussions of DRF questions in the class. |
|
||
| 12. Our classmates can help me to sole learning difficulties in DRF class. |
|
||
| 13. Our classmates are imbued with learning atmosphere in DRF class. |
|
||
| 14. Our classmates can share and cooperate with others' opinions in DRF class. |
|
||
|
|
15. I can actively set out learning schedule of the DRF class. |
|
|
| 16. I will take previews of our DRF texts before class. |
|
||
| 17. I will take reviews of our DRF texts before class. |
|
||
| 18. With applications of DRF effective learning, I can pay more attention to study. |
|
||
| 19. I can do my best to complete DRF assignments by our instructor. |
|
||
| 20. I think DRF teaching can upgrade my scores in the class. |
|
||
| 21. Our DRF-integrated teaching methods can enhance my macroscopic problem-solving abilities. |
|
||
| 22. Our DRF-integrated teaching methods can enhance my microscopic problem-solving abilities. |
|
||
| 23. Our DRF-integrated teaching methods can increase my willing to pursue new knowledge. |
|
||
| 24. Our DRF-integrated teaching methods can inspire my willing to pursue new knowledge. |
|
||
| 25. I completely agree to integrate our DRF teaching methods into chemistry learning. |
|
||
| KMO=0.943 Accumulative Explanation Variation(%)= 65.341 Total Cronbach's É = 0.959 |
|||
To meet strategic applications of DRF teaching modules, this research explored whether there were any significant statistical results between the experimental group students and the control group students. Statistical analyses were treated for students' posttest learning achievements, with students' pretest data as covariate variables, and posttest data as dependent variables, and two divided groups as independent variables. Homogeneity examinations of the regression slopes showed that there were no significant statistical differences between two group students for the unit of redox chemistry learning by independent variables and dependent variables, responding to group assumptions of covariate variable analyses. Therefore, further covariance analyses were available for this research. Statistical results of covariants listed in Table 2 indicated that there were significant differences in students' posttest achievements between two groups. The result that the Cohen's experimental effect size (f), f value were .61 indicated a higher effect size in the unit of redox chemistry learning. The posttest scores of the experimental group were higher than those of the control group, which confirmed the major assumption that the strategy of experimental teaching was better than that of traditional teaching.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*** p< 0.001
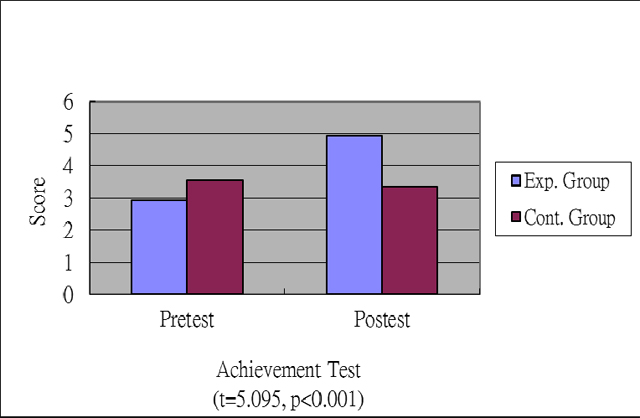
This study put more emphases on pairwise comparisions with
posttest mean values so that t-test rersults showed that students'
posttest scores of the experimental group were higher than those
of the control group, as shown in Fig.
2. After teaching implements of posttest scores' covariance,
pairwise comparisons, and learning achievement tests, all DRF
statistic applications had more significant influence on students'
learning achievements. More significant differences between two
student groups in the unit of redox chemistry learning were detected.
The result for statistic differences was attributed to the fact
that highlighted DRF chemistry with systematic knowledge structure
and repeated animation presentations, not only showed macroscopic
differences in students learning of chemical reactions, but also
presented changeable factors of microscopic conceptions of ions
and charge transmissions. Presentations of highlighted DRF chemistry
gave students learning interactions to analyze, compare, criticize,
feedback, link symbols and abstract conception relationships between
microscopic ions and charge transmissions with accurate recognizable
knowledge.
To complete the presentation of integrated DRF chemistry, this
study made surveys of students' satisfactory in learning for a
better statistic module of students' chemistry learning. After
statistical analyses of experimental teaching, this research chose
experimental group students as students' satisfactory in learning
for statistical analyses. The statistic results of students' satisfactory
in learning in Table 3 showed
three dependent variables (Q1, Q2, and Q3) and total correspondents
of mean values (M), standard derivation values (SD), and Cronbach's
_ values (_) in the unit of redox chemistry learning. The overall
Cronbach's _ values (total) was 0.989, indicating that the internal
consistency of retest total scales reached a satisfactory degree
[26], and their mean values were
over 3.19, indicating that after a series of experiments students
in the experimental group had more positive satisfactory in chemistry
learning.
Statistical analyses of learning satisfactory attitudes, with
the three-subscales of students' satisfactory in learning as dependent
variables, this study chose students' gender, enrolment, disposition
toward chemistry, and frequency of the digital-modules usage as
independent variables for statistical analyses of one-way ANOVA
to explore if there existed any significant changes in the multi-
variables of the Wilks' Lambda parameter. All multi- variables
significances in Wilks' Lambda parameter were listed in Table
3, including the F-ratio, p-value, and effect
sizes (f).
Statistical results of students' independent variables for disposition
toward chemistry in the unit of redox chemistry learning reached
significant differences. For further Scheffes' post hoc comparisons,
this research found out that the positive disposition in the subscale
Q1 was larger than the negative disposition, the positive disposition
in the subscale Q2 was larger than the negative disposition, and
that the positive disposition in the subscale Q3 was larger than
the neutral and negative dispositions. Results of effect sizes
in three subscales were 0.87, 0.51 and 0.49 respectively, indicating
larger effect sizes (f > 0.4) [27].
Learning attitudes for students' independent variables of disposition
toward chemistry were shown in subscales Q1, Q2, and Q3. No significant
differences for independent variables of gender, enrollment, and
digital module frequency were detected in redox chemistry learning.
Statistical results of three subscales were indicated from small
(f is 0.1) to large (f is 0.4 above) effect sizes [27] (in Table
3).
|
|
|
|
Attitude | Measure | |
| Q1 | Q2 | Q3 | |||
|
|
(male, female) |
F-ratio | 0.477 | 0.076 | 0.385 |
| p-value | 0.493 | 0.784 | 0.538 | ||
| f | 0.11 | 0.15 | 0.12 | ||
|
(grades,recommendation, application, no test) |
F-ratio | 0.018 | 0.701 | 0.114 | |
| p-value | 0.997 | 0.557 | 0.951 | ||
| f | 0.28 | 0.15 | 0.26 | ||
|
|
F-ratio | 10.124 | 3.739 | 8.495 | |
| p-value | 0.000*** | 0.032* | 0.001** | ||
| f | 0.69 | 0.42 | 0.64 | ||
|
|
F-ratio | 0.520 | 0.569 | 0.290 | |
| p-value | 0.671 | 0.639 | 0.833 | ||
| f | 0.19 | 0.18 | 0.23 |
*p<0.05; **p<0.01; ***p<0.001
In short, most students firmly agreed that applications of DRF chemistry technologies were helpful for clarifying the process of ions and charge transmissions and increased students' learning interests and skills in problem-solving. It was expected that learners had the strategic agreement and usefulness of DRF chemistry technologies because of the implements, demonstrations and multi-functions in problem-solving strategies.
To be a promising strategic teaching, this study incorporated computer animated presentations into DRF problem-solving abilities with suitable interviews for promoting students' chemistry performances in the following way.
The statistical analyses of this research explored implemented validity of DRF problem-solving skills in previous research results [15, 28-30]. While traditional lecture-based teaching could not fully meet students' learning and curiosities, this study recommended multimedia DRF problem-solving strategies to promote learners' chemistry learning performances. Statistical results showed that experimental group students' learning performances, with strategic abilities of DRF, had higher posttest scores than those of controlling group students. The same experimental group students with strategic abilities of DRF had more significant and increasing learning achievements in posttests of problem-solving skills in the chemistry learning unit than in their pretests. For different dispositions of chemistry, experimental group students also indicated more significant satisfactory in chemistry learning, and over larger effect sizes (f > 0.4) in the unit of redox chemistry learning. According to semi-structure interviews between students and teachers, all DRF chemistry applications had much appeal to students' curiosity and interests, which could enrich their satisfactory in learning and construct the validity of concise chemistry conceptions.
This research is aimed at the validity of DRF problem-solving
tools in promoting students to have the macroscopic and microscopic
demonstrations with symbols and to clarify students' overall conceptions
in chemistry, as well as in enhancing their problem-solving skills
and learning performances. Three further suggestions could be
indicated below:
(1)Strengthening more highlighted DRF applications
The highlighted DRF chemistry should be designed to contain more
practices and demonstrations, including textual explanations,
static figures, and colorful presentations in order to attract
students' learning motivation and good cognition.
(2)Building up a well-equipped e-environment in chemistry learning
Educators should set up digital multimedia equipment with projector
slides and well-prepared DRF presentations.
(3)Linking macroscopic and microscopic demonstrations with chemical
symbols
Students were required to link chemistry conceptions between verbal
and visual inputs and to construct macroscopic and microscopic
DRF abilities with chemical symbols.
The author would like to thank journal editors and the anonymous reviewers of this paper for their kind assistances and helpful suggestions. A short but sincere thank must also be given for the patronage of the National Science Council in Taiwan (under Grant No. NSC 99-2511-S-237-002 & 101-2511-S-237-001). Without all their help, this study could not have been completed in present form. Finally, thanks must also be given to all the instructors and students who participated in this research study.