2Department of Science Education, National Taipei University of Education, Taipei, Taiwan
3Department of Chemistry, Capital Normal University, Beijing, China,
E-mail: dongsujing
Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-105 / Received August 30, 2013.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
Chemistry is an important part of science, and labwork is
the central part in teaching the course of chemistry in high school.
With the aim of analyzing labwork of chemistry textbooks in high
school in Beijing and Taiwan and on the basis of 'A map of labwork'
in Labwork in Science Education (1998) working paper 1 and working
paper 3, this study emphasizes on comparing and analyzing the
learning objective and features of task. In the study we use the
definition of science literacy in PISA framework 2009 to analyze
the chemical experiment designed in the laboratory manual. Under
one guiding principle there are various chemistry textbook editions
in Taiwan. We use the Hanlin (HL) edition recommended by high
school teachers of chemistry in Taiwan as a research object and
compare them with those published by the People's Education Press
(PEP) used by most of the high schools in Beijing. Through the
comparison and analysis of the intended learning objective and
features of task, teachers will come to a better understanding
of what kind of influence their teaching of chemistry experiments
can make on students' learning of chemistry and on their future
development.
Keywords: Labwork, high school chemistry curriculum,
Science literacy
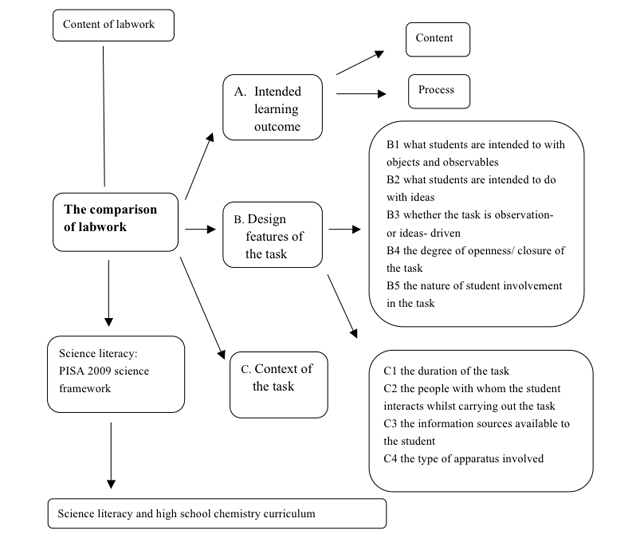
Dimension A: Intended learning outcome (learning objectives)
Dimension B: Design features of task
What students are intended to do with objects and observables (B1)
What students are intended to do with ideas (B2)
Observation or ideas driven (B3)
Dimension C: Details of context
People whom students interact with (C2)
Beijing and Taiwan have very similar cultural background and use the same language, but still there are differences in some areas. For example, in the field of chemistry education of high school, the formulation of curriculum criterion is subject to influences from the differences. After the new round of high school chemistry curriculum reform carried out by Beijing in 2007, the new chemistry textbooks published by People's Education Press (PEP) have played an essential role on improving science literacy of students and promoting their comprehensive development. Taiwan has made gratifying results in the tests of Program for International Student Assessment (PISA) carried out by Organization for Economic Co-operation and Development (OECD) and Trends in International Mathematics and Science Study (TIMSS) carried out by the International Association for the Evaluation of Educational Achievement (IEA), which examined comprehensively the science literacy of students, and measured the capabilities they need in making decisions related to science and technology in the future.[1,2]
We use A 'map' of 'labwork' from "Labwork in Science Education"
by Millar et al. [3] to make
a comparison. Also, the definition of science literacy in this
research is quoted from PISA 2009 science framework.
High school teachers both in Beijing and Taiwan are also invited
to analyze the experiments.
The framework of the research is shown in Figure
1. [3]
|
|
|
|
|
|
|
Chapter 1 Learning chemistry through labwork 1. Filter and Evaporate |
Chapter 1 The structure of matters and periodic law 1. The property of alkali mental |
|
Chapter 2 Chemical substance and its change 6. The property of colloids |
Chapter 2 Chemical reaction and energy 6. Chemical energy and heat |
|
Chapter 3 Metal 9. The property of Na and Al |
Chapter 3 Organic compounds 10. The substation reaction of CH4 |
|
Chapter 4 Non-metal
|
Chapter 4 The development and utilization of natural resource and chemistry 17. Thermite reaction |
|
|
|
|
Foundational chemistry 1 Chapter 1 The composition of matter |
Chapters without experiments Chapter 3 Chemical reaction |
|
Foundational chemistry 2 Chapter 1 The structure and property of matter |
|
|
Foundational chemistry 3 Chapter 2 Chemical reaction rate |
Chapter 1 GasChapters without experiments |
Chemical labwork is an important part of chemistry learning, and the contents of labwork and chemistry textbooks are closely related. Here we give a brief account of the contents of labwork and chemistry textbooks both in Beijing and Taiwan, as shown in Table 1 and Table 2.
There are two compulsory textbooks containing eight chapters, 42 experiences and six other selected textbooks published by PEP in Beijing; it takes one school year for Beijing students to learn the two compulsory textbooks. [9]And there are three foundational textbooks containing 11 chapters, 12 experiences and two other selected books published by Hanlin Press in Taiwan. It takes Taiwan students two school years to learn the three foundational textbooks. [10] The content of selected textbooks is not taken into consideration. Beijing students have much more labwork classes than Taiwan students do.
PEP 1 means the compulsory chemistry 1 published by People's Education Press; and PEP 2 means the compulsory chemistry 2 published by People's Education Press. HL 1 means the foundational chemistry 1 published by Hanlin Press, HL 2 means the foundational chemistry 2 published by Hanlin Press, and HL 3 means the foundational chemistry 3 published by Hanlin Press.
The teaching or learning tasks generally start with the establishment of the learning objectives by teachers, which leads the teachers to make further teaching plans to achieve the goal. In this part, chemistry experiments in high school textbooks are classified according to the specified experiment objectives. The learning objectives are classified into two categories, one is about the learning of science content and the other is about the processes of scientific enquiry. [3] Further classifications are shown in table 3.
In A-b, a 'fact' means factual knowledge of chemistry, which is closely related to the quality of substances, and reflects a wide-ranging knowledge such as the presence, manufacturing method, preservation, utilization, test and reaction of substances. Factual knowledge in chemistry consists of two major parts, knowledge of inorganic elements and compounds and knowledge of organic compounds.
In A-d, a 'relationship' might be a pattern or regularity in the behavior of a set of objects or substances, or an empirical law, such as Periodic law, the factors affecting the chemical reaction rate, Le Chatelier's Principle and so on. [11,12]
In A-h, 'To make an experiment design to address a specific
question or problem'£¨is to help students grasp basic
scientific methods and apply the scientific methods in chemistry
experiments.
e.g. Students design the 'control variables' experiment, or
learn how to make the ion (Cl-, SO42-) tests. [4]
In A-i, when students need only to process experimental data
and calculations, this kind of experiments are categorized A-i.
e.g. Utilize colorimetry, that is to make iron ions (Fe3+)
react with thiocyanate ions (SCN ), to measure the concentration
of the product iron thiocyanate ions (FeSCN2 +),
so as to obtain the equilibrium constant Kc. [12]
In A-j, the 'data' includes both experimental phenomena and experimental data. Students need to record the experimental phenomena and data and explain the experimental phenomena, then come to a conclusion or write down chemical reaction equations. This type of experiments is categorized A-j.
The classification is based on the learning objectives of laboratory
manual.
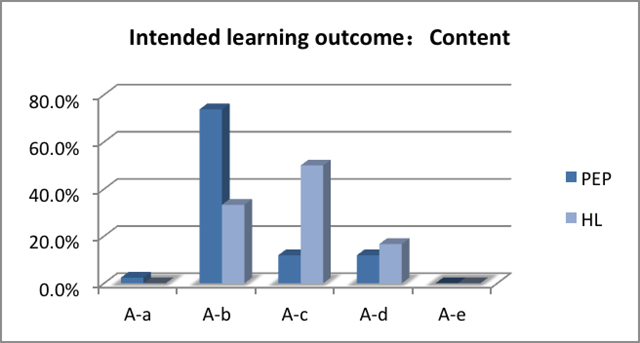
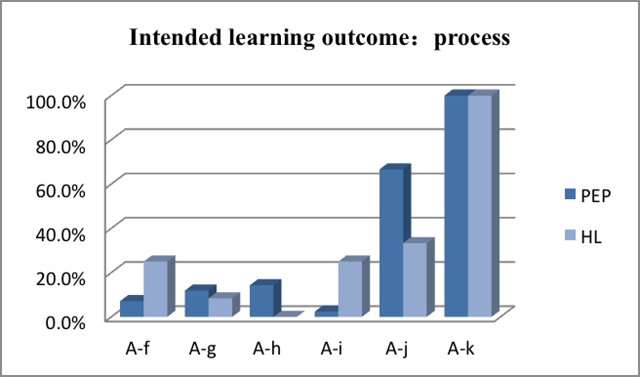
Figure 2 and Figure
3 separately show the learning objectives that are related
to the learning content and processes and the percentage of the
number of experiments corresponding to the PEP learning materials
and HL learning materials under each specific experiment objectives.
Through comparison, we can see the main learning objective of
chemical experiments is to help students 'learn factual knowledge',
which includes understanding the properties of substance or the
basic principle. In Beijing, helping students 'learn factual knowledge'
in chemistry experiment courses, which means the learning of properties
of substances or materials, takes a large percentage of all labwork,
as shown in Table 1. However, the
main purpose of doing chemistry experiences in Taiwan is to help
students 'learn a concept', such as dissolution, crystallization,
or solubility product constant, as shown in Table
2.
The learning objective related to the process, both in Beijing and Taiwan, is to help students share their reports or have better discussions, for students are required to accomplish their experimental reports and write down the experiences they gain through the experiments. Also, the textbooks emphasize on helping students both in Beijing and Taiwan 'learn how to use data to support a conclusion'. As for the requirements to students, there are differences between Beijing and Taiwan. The students in Taiwan need 'to learn how to use rightly a standard laboratory instrument, or to set up and use a standard piece of apparatus', but students in Beijing need 'to learn how to plan an experiment to address a specific question or problem'. Of all the experiments, this type of experiments covers a large proportion. For example, when students in Beijing are intended to design the experiments to inquire the substation reaction of CH4, they must learn how to control variables first. [13] In Taiwan, however, when students learn the 'general property of organics', one of the learning outcomes is to learn the 'use of a dropper and a cylinder correctly'. [7] Also, it is significant that students in Taiwan learn 'how to process data' through labwork.
|
|
|
|
|
|
|
A-a to identify objects and phenomena and become familiar with them A-b to learn a fact or facts A-d to learn a relationship |
A-f to learn how to use a standard
laboratory instrument, or to set up and use a standard piece
of apparatus A-g to learn how to carry out a standard procedure A-h to learn how to arrange an investigation to address a specific question or problem A-i to learn how to process data A-j to learn how to use data to support a conclusion A-k to learn how to communicate the results of their work |
PEP means the experiments shown in chemistry laboratory manuals by People's Education Press in Beijing, and HL means the experiments shown in chemistry laboratory manuals by Hanlin Press in Taiwan.
The second main dimension of the map aims at characterizing the design features of labwork tasks: what students are intended to do with objects and observables (B1) and what they are intended to do with ideas (B2). [3] The further sub-division is shown in table 4 and table 5.
This sub-dimension of the map (B1) relates to what students are intended to do with objects and observables. [3]
Most labwork tasks involve the student in manipulating and/ or observing objects or materials. Some involve learning how to use instruments rightly, or procedure of basic laboratory operation. The students may be intended to:
A different type of task is one which involves learning how to present an object so as to display certain features of it clearly. Here the student is intended to:
Other tasks require the student to make something. This may be a physical object, or a material. [3]
The fourth, and perhaps the largest, category of labwork tasks is those which require the student to observe something. The observation may be of an object or a material. The student may be intended to:
In other situations, the observation is better characterized as observation of an event. Here the students are required to:
(Demonstration experiments that students are required to record experimental phenomena.)
Finally, the task may involve the observation of a physical quantity (or variable) associated with an object, or material, or event. Such an observation may be qualitative (e.g. an observation of color), or semi-quantitative (noting if something is large, or small), or quantitative (i.e. a measurement). In these cases, the student is intended to:
The analysis is based on the interview to high school chemistry teachers. Also the laboratory manuals and chemistry textbooks which describe the process of each experiment are taken into consideration.
It is significant that students are intended to 'make events occur' both in Beijing and Taiwan. According to the arrangement of the chemistry textbook published by PEP, more than 70% of the experiments are designed to let students make events including reactions occur. However, this kind of labwork in Taiwan's chemistry textbooks published by Hanlin takes about 40%. Students in Taiwan are intended to do more of observation and other type of tasks than students in Beijing do.
The result is shown in Figure 4 which indicates the percentage of each class of experiments with specific content representing what students are intended to do with objects and observables.
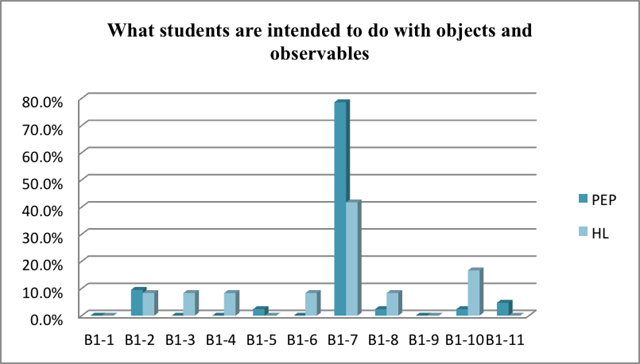
| use | an observation or measuring instrument | B1-1 |
| a laboratory device or arrangement | B1-2 | |
| a laboratory procedure | B1-3 | |
| Present or display | an object | B1-4 |
| make | an object | B1-5 |
| a material | B1-6 | |
| an event occur | B1-7 | |
| observe | an object | B1-8 |
| a material | B1-9 | |
| an event | B1-10 | |
| a quantity | B1-11 |
This sub-dimension of the map (B2) is related to what students are intended to do with ideas.
Labworks are not only the observation or operation of object, but also to allow students to practice their ideas, improve their knowledge structure and even inspire more inspiration through experiments. This means that labwork is 'hands-on' as well as 'with the brain thinking'. Undoubtedly, labwork is the bridge connecting theory and practice, representation and concept. Therefore, a labwork task can also be classified according to what the students are intended to do with ideas. [3]
Some labwork tasks simply require direct reporting of observations, though the selection of features to observe and record is inevitably influenced by the teacher's and/ or student's purposes and understandings:
Other labwork tasks require the students to identify a pattern, or regularity, in the behavior of the objects or events observed:
One particular type of 'pattern' which is common (and so worth
keeping as a separate category) is the relationship between experiment
objects, or between physical quantities (variables). Students
may be asked to:
Another type of labwork task is one which is designed to help
students to develop their ideas by seeing that a new concept,
or quantity, can help them to interpret their observations. However,
this type of labwork task is not involved in textbooks both in
Beijing and Taiwan.
Another type of labwork task involves the testing of predications.
A prediction may be simply a guess, or may be deduced from a more
formal understanding of a situation, such as an empirical law,
or a theory (or model). In labwork tasks of these sorts students
are intended to:
Finally, some labwork tasks are about accounting for observations, either by relating them to a given explanation or by proposing an explanation. [3] An 'explanation' might be an empirical law, or a general theory, or a model derived from a general theory, or general principles derived from a theoretical framework. In some tasks, the explanatory ideas are known in advance and the student is expected to use these to account for what is observed, perhaps extending or modifying the framework of ideas.
A variant of this is where two (or more) possible explanations are proposed and the task is to decide which accounts better (or best) for the data. In other tasks, the observations come first, and the student is expected to select an explanation from his/her existing knowledge, or perhaps to extend this to develop an explanation.
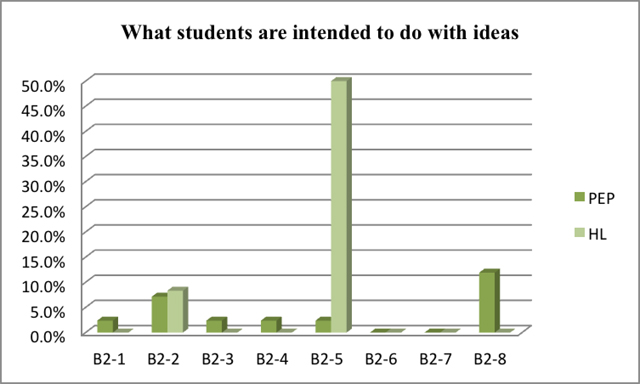
As seen in Figure 5 and Figure 6, students both in Beijing and Taiwan are supposed to 'accounting for observations in terms of a given law'. It is significant that students in Taiwan need to 'explore relations between objects and physical quantities', which takes the percentage of nearly 50%. In Beijing, however, students are required to 'account for observations by proposing a law', and 'test a prediction from a law or from a guess'.
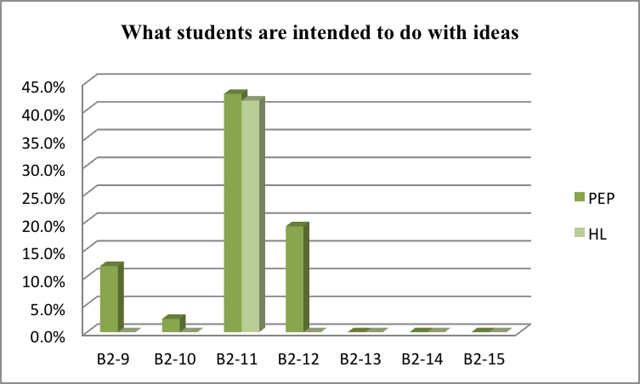
| direct reporting of observation (s) | B2-1 | |
| identify a pattern | B2-2 | |
| explore relation between | objects | B2-3 |
| physical quantities | B2-4 | |
| objects and physical quantities | B2-5 | |
| 'invent' a new concept (physical quantity, or entity) | B2-6 | |
| determine the value of a quantity which is not measured directly | B2-7 | |
| test a prediction | from a guess | B2-8 |
| from a law | B2-9 | |
| from a theory | B2-10 | |
| account for observations | in terms of a given law | B2-11 |
| by proposing a law | B2-12 | |
| in terms of a given theory | B2-13 | |
| by proposing a theory | B2-14 | |
| choose between two (or more) explanations | B2-15 | |
Take 'chemical energy and heat' for example, students in Beijing would make an interpretation according to the phenomenon of the change of reaction temperature, which is 'accounting for observation by proposing a law'. The same experiment in Taiwan, however, requires students to prepare a specific solubility solution precisely following the procedure and record the temperature change with the reaction occurrence, which help students explore relations between objects and physical quantities.
The result is showed in Figure 5 and Figure 6 which indicate the percentage of each class of experiments with specific content representing what students are intended to do with ideas.
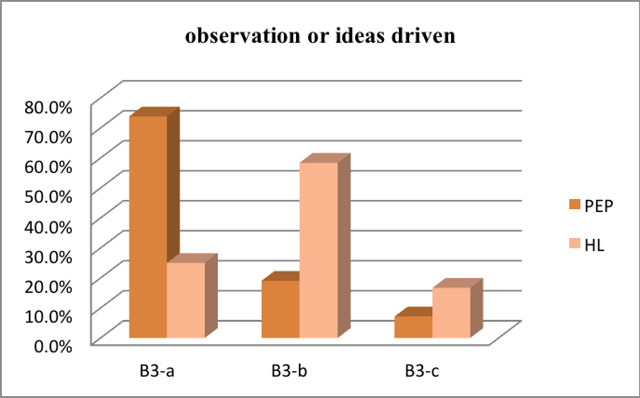
Most experiments read in chemistry textbooks published by PEP are object driven, which means 'what the students are intended to do with ideas arises from what they are intended to do with objects'. On the contrary, most labworks showed in chemistry textbooks published by HL are ideas driven, which means 'what the students are intended to do with objects arises from what they are intended to do with ideas'.
The result is shown in Figure 7 which indicates the percentage of each class of experiments with specific content representing whether an experiment is observation driven or ideas driven.
|
B3-a What the students are intended to do with ideas arises from what they are intended to do with objects B3-b What the students are intended to do with objects arises from what they are intended to do with ideas B3-c There is no clear relationship between what the students are intended to do with objects and with ideas |
This categorization aims at showing how to take the initiative in labwork activity. Figure 8 and Figure 9 show the modules of laboratory manuals in Beijing and Taiwan separately. We can figure out students in Beijing have higher degree of participation in chemistry experimental courses, which means they could choose the apparatus and discuss the procedure with peers or teachers. Students in Taiwan, on the contrary, just need to follow the procedure rather than design the experiments. Students both in Beijing and Taiwan are required to record phenomenon and data of the experiments and finish the reports. We should notice that chemistry textbooks published by Hanlin Press in Taiwan emphasize more on waste disposal. Textbooks published by PEP in Beijing, however, pay more attention on applying the principle or reaction in our daily life.
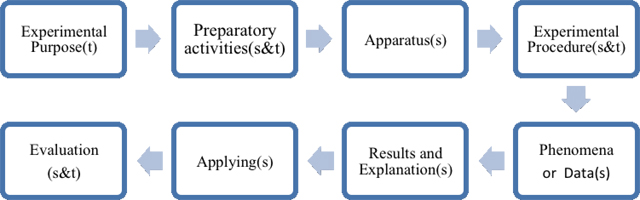
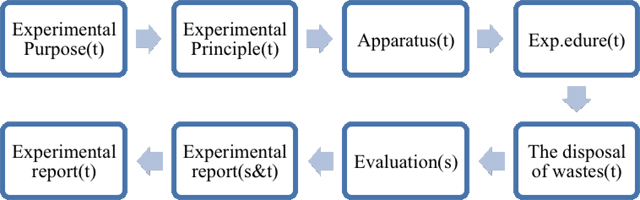
| s = chosen by students |
| t = written in the textbook |
| s&t = decided through teacher- student discussion |
This aspect deals with the level of student involvement: as observer, as assistant, or as executor of the task with other students or on one's own. According to chemistry curriculum standard developed by the Ministry of Education published by PEP in Beijing, there is not a clear boundary between students' experiments and demonstration experiments, which depends on the condition of the lab or the schedule. In Taiwan, however, there are 12 experiments, including 10 students' experiments, in which students act as executors of the task, and two demonstration experiments, in which students act as assistant or as observer.
There are many factors that may affect the result of an experiment, such as whether students have enough time to complete the labwork; whether they can get help or discuss with someone when they have a problem during experiments; or if students have the opportunity to learn from the failure. Therefore, it is important to know some details about the context, and this dimension consists of the following four parts [14]:
The information of the details of context is from the interviewing to chemistry teachers in Beijing and Taiwan.
In Beijing, each experiment is usually less than one class (40 minutes), but in Taiwan, each experiment lasts for one class (50 minutes). There is always a deeper discussion about the experiment in Taiwan in the next class, and teachers help students build the bridge between the theory and practice. In Beijing, however, students always get the conclusion or learn something new directly from the experiment, and teachers review the knowledge in the next class.
Students in Beijing prefer to discuss with their classmates carrying out the same labwork task, and when they fail to get a result and the phenomenon is different from what they expected before, they will ask the teachers for help. Similarly, students in Taiwan would like to interact with other students carrying out the same labwork task or students who have already completed the labwork task and teachers or more advanced students (demonstrators).
Both in Beijing and Taiwan, students get information source through textbooks, rather than computers or mobile phones in class.
In Taiwan, some teachers confirmed in answering a question that students use standard laboratory equipment, and some students are trained and volunteer to prepare the apparatus for experimental course. In Beijing, however, teachers will prepare the apparatus and students just do the experiments.
In Beijing, most experiments are designed to help students learn facts or the property of materials that are the important parts in chemistry learning, and in most cases, students are required to use data to support a conclusion. During the experimental course, students have to make chemical reactions occur, and then they usually need to get the conclusion by accounting for observations in terms of a given law or accounting for observations by proposing a law, and usually explore the idea according to the operations on objects. More importantly, students are involved in the design of the procedure, and they learn directly from labwork and try to explain some phenomena occurred in daily life. Students in Beijing learn the property of common material in life through chemistry labwork and gain the capability of explaining and analyzing what they observe. Almost every experiment class is of short duration, but the labwork has turned out to be an indispensable part of the chemistry teaching. Thus we can come to the conclusion that labwork in Beijing may improve students' science literacy [1] which consists of the following three parts:
Chemistry experimental class of high school in Taiwan is similar with that in Beijing, but there is a little difference. Students in Taiwan learn concepts through labwork and then apply these concepts to the chemical experiments. The purpose of the labwork is to help students use the data they obtained through the experiments to support the conclusion and use the laboratory instruments correctly or set up and use the apparatus in the laboratory. Also, some of the students are able to help teachers prepare the apparatus in advance for other students. Students also explore relations between objects and physical quantities in the experimental class. And what's more, students learn the principles ahead of the experiments, so what they need to do is to follow the procedure as criterion or recipe. Students in Taiwan pay more attention on concepts learning and calculation. When students meet troubles in experiments, they can discuss with other students and teachers, and when they fail in an experiment their teacher would give them a chance to redo it. Thus students in Taiwan have more chances to learn from failure in comparison with students in Beijing. From the chemistry textbooks published by Hanlin Press in Taiwan, we can see Taiwan's chemistry labwork teaching focuses more on helping students acquire scientific knowledge.
The chemistry education both in Beijing and Taiwan have managed to make students:
This study is only involved in the teaching and learning of chemistry in high school in Beijing and Taiwan. Chemistry education in junior high school on both sides is not considered. There is a slight difference between Beijing and Taiwan in chemistry education in junior high school, which may have some influence on students' learning of chemistry in high school.
Due to various constraints, the study is mainly dependent on chemistry textbooks, chemistry laboratory manuals, interviews with teachers of high school and classroom observations. But the study may not be that perfect for lack of interviews with high school students both in Beijing and Taiwan.