Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-106/Received July 28, 2013.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
The present condition of teacher education in Japan and the
development of the training program with experiment study are
introduced. Beginning in March 2009, MEXT (the Ministry of Education,
Culture, Sports, Science and Technology) enforced a system of
"certificate renewal course" that required educators
to acquire the advanced knowledge and skills every 10 years in
cooperation with a university. MEXT has also begun a comprehensive
review of policies to improve the quality of in-service teacher,
including enhancement of pre-service teacher training courses
in universities. The board of education under jurisdiction of
each prefecture has also enforced in-service teacher training
originally. The challenges as Tokyo Gakugei University and as
the department of chemistry to pre- and/or in-service teacher
training were made. The project learning programs and the experiment
learning programs have been developed.
Keywords: Teacher education, Certificate renewal course,
Experiment learning program, Subject of "Science subject
research", Method of instruction
Learning program of Tokyo Gakugei University as an example
Liberal arts subject of "Project learning" for all the student
Challenges of teacher education for pre- and in-service in Japan have so far been made for improvement of the quality of teachers by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) [1] and the board of education under jurisdiction of each prefecture by the guidelines to elementary and secondary education [2]. MEXT has also begun a comprehensive review of policies to improve the quality of teachers, including enhancement of pre-service teacher training courses in universities. We have developed an experiment learning program for science teacher education, and practice of some programs has been carried out to students of teacher training and/or in-service teachers. In this paper, the current situation of teacher education in Japan and some trials of the training program with experiment study are introduced.
Beginning in March 2009, MEXT enforced a system in cooperation with a university for "certificate renewal course" that requires educators to acquire the most advanced knowledge and skills every 10 years, where educators need to have short course training for 30 units equivalent in 30 hours including 12 units or more as the matter about the educational newest situation plus 18 units or more as the course instruction about the matter about student instruction and other substantial education. In the case of 2013 fiscal year for the system, 465 authorized universities and corresponding organizations take part in the short course training area of 303 requirements (R) and 453 selections (S). The number of short courses becomes to be Totally 751 R and 7,131 S for acceptance schedule with number of candidate teachers of ca. 100,000 of each R or S.
On the other hand, in each jurisdiction prefecture, the board of education has already carrying out the original teacher training for elementary and secondary school teachers in accordance with the guidelines. In the case of Tokyo Metropolitan Board of Education [2], school personnel in service training center requires compulsory training of young faculty for development training, e.g. the training needs 180 hours in the inside of a school plus 16 days in the outside of a school for the first year teacher, 30 hours plus 1.5 days analogues for the second year teacher, 30 hours plus 1 day analogues for the third year teacher, and 16-24 units for every 10 year teacher. Optional training is also set up for professional training as like the themes of "Development of teaching materials on the DNA (organism)," "Teaching methods in the contents of the radiation device (physical)," etc.
Tokyo Gakugei University (TGU) is one of the national universities of independent administrative agency in Japan and has a reputation of Japan's center of teacher education. Programs of student education consist of Teacher Training Course and Liberal Arts Course in faculty of education, Graduate School of Education (Master's Course), and United Graduate School of Education (Doctoral Course) [3]. Curriculum of teacher training course of bachelor's degree consists of liberal arts subjects, foundation subjects, content subjects, and graduation research positioning as a compilation (goal) of an educational program (Figure 1).
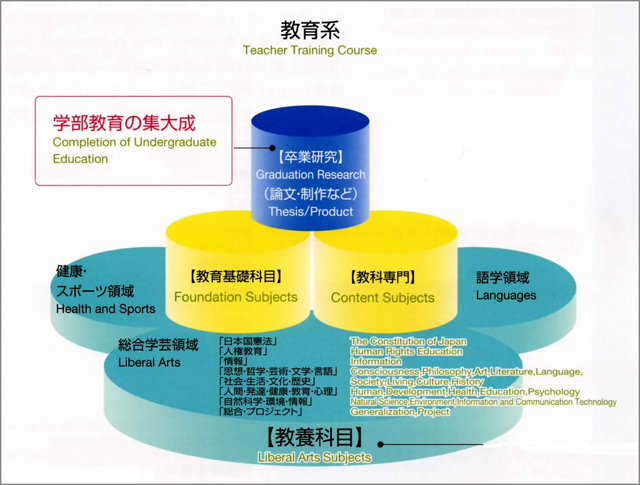
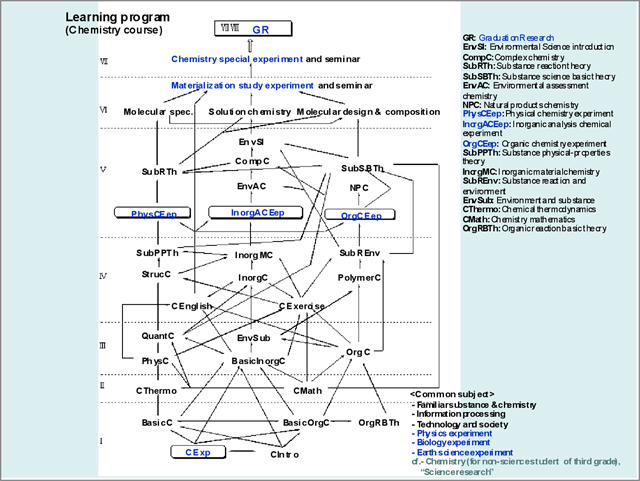
In the case of learning program for chemistry course student, sufficient lectures and experiments are required for graduation research as completion of undergraduate education, where four experiments as a content subject of "Chemistry experiment," "Physical chemistry experiment," "Inorganic chemistry experiment," and "Organic chemistry experiment," as a compulsory experiment are imposed besides three experiments as a foundation subject of "Physics experiment," "Biology experiment," and "Earth science experiment" obligated in science course (Figure 2).
Liberal arts subject of "Project
learning" for all the student
Subject of "Generalization-Project" is usually referred
to as "Project learning" (Figure
1). Policy is stated as follows, project learning consists
of buildup approaches as preliminary quasi-graduation research
for second grader in one year by three to four professors' support
through scientific methodology [4].
Three compulsory subjects are set up as "Project study subject
I," "Project study subject II," and "Comprehensive
exercise." The research activities including extracurricular
activities for a half or one year are expected. Seven fields are
set up for all the students, and there are "Science and Technology"
and "Environment" as a field about science. Twenty eight
themes in 2009 were set up, and eight examples related with science
were stood as a candidate as follows,
- Method of natural science
- Modern society and charge of a substance
- Present and the future of life science
- Space earth science using educational establishment or teaching
materials
- Robot contest
- Preservation and practical use of cultural assets
- Environmental education in the outdoors
- History of nature of Tama River.
Each student chooses and studies one theme which is separately pleasing. EachÅ@theme is put weight on inquiry-based learning as the preparatory step towards graduation research. Typical methodology of science by scientists including the procedure of integration of results, hypothesis, modeling, and verification should sustainably be repeated and repeated, as much as any other profession. Even in the subject of "Project learning" it is desirable that students should educate themselves in a similar manner with their tolerance through research.
Experiment subject for chemistry
course student
Four experiments of "Chemistry experiment," "Physical
chemistry experiment," "Inorganic chemistry experiment,"
and "Organic chemistry experiment," as a compulsory
experiment are imposed besides three experiments of "Physics
experiment," "Biology experiment," and "Earth
science experiment" obligated in science course (Figure
2).
Policy to the subject The experiments proceed typically
as a manner of the following flow of the lesson as foundations;
- Step 1. Data distribution about an experiment (beforehand)
- Step 2. Basic and deployment experiments
- Step 3. Arrangement of results- Robot contest
- Step 4. Report creation (home work)
- Step 5. Interview (office hours, individually)
- Step 6. Acceptance of report.
Each step is able to retry for student as extracurricular activity
following designated lesson time and/or at office hour.
Student's lab through experiment program is set up in consideration of the viewpoints of
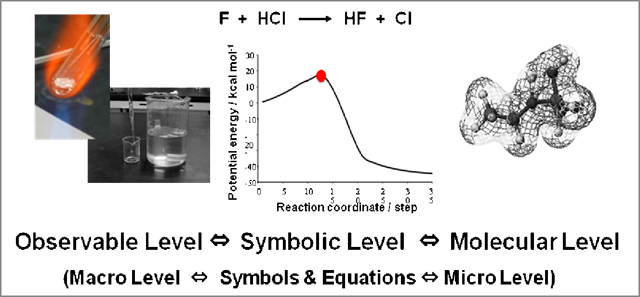
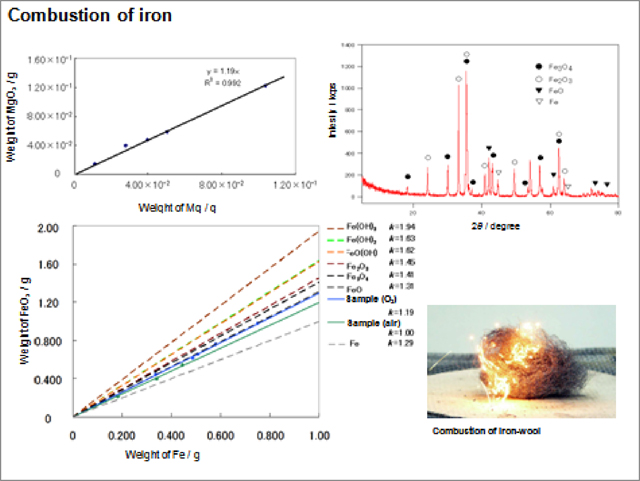
three thinking levels of observable level, symbolic level, and molecular level, respectively (Figure 3). Dividing the lecture stage into the three thinking levels was mentioned by Tasker et al. [5]. Student's lab in our experiment learning program proceed mainly at observable level besides at symbolic level and molecular level based on offer data, search data, and/or knowledge acquired from the outer opportunity, e.g. in the lab of "Combustion of iron" students do the experiment of combustion of iron-wool and measure the XRD patterns at observable level, and they make the graphs by single regression curve processing based on the data and those of postulated substance at symbolic level (Figure 4). Acquisition of the image to the target chemical reaction is great help for student to have images of phenomena, chemical concepts, and molecular world. Students' enthusiastic activities with imaginative thinking and behaving are expected in the student's lab.
Through the theme of "Dissolution of salt" Dissolution of salt of NaCl and KCl is an endothermic reaction. The reaction is not easy for student to understand why the reaction proceeds, and the reaction leads to confusion or misconception from a thermodynamics standpoint because of the ambiguous definition of energy in secondary education level, i.e. a student learns that a chemical reaction occurs toward the direction of low energy in a spontaneous reaction. The reaction profile in figures cited in current textbooks is expressed with energy by the vertical axis and with a reaction coordinate by the horizontal axis, in which energy is only defined as "energy" further with an ambiguous expression or non-description about a reaction coordinate in many cases.
Students measure the heat of sodium chloride NaCl and/or potassium chloride KCl dissolution in water at 298 K by infinite dilution method through laboratory work and calculate enthalpy change of the dissolution ΔHdissol. Students search literatures of enthalpy change of lattice ΔHlat simultaneously with the dissolution experiment. Original diagram of dissolution processes about NaCl developed by our quantum chemistry calculation is provided to students as reference data (Figure 5). Furthermore, the intelligible diagram is represented by the thermodynamic changes of Gibbs function ΔG. Students come to realize by the standpoint of observable level of the experiment and understand by those of symbolic level and molecular level meaning of the thermodynamic changes in the phenomenon. On extension of the experiment, the number of hydration should be calculated by the entropy method with total enthalpy change of the hydration ΔHhyd.
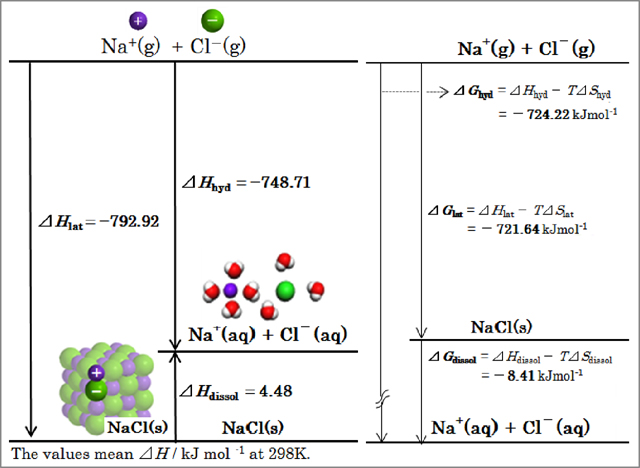
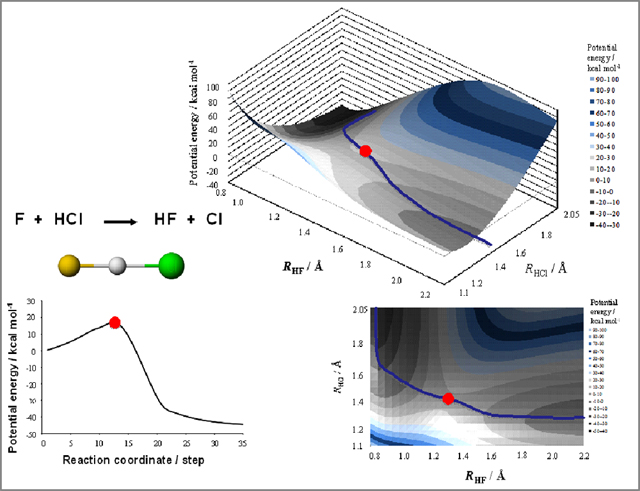
About "reaction coordinate" with an ambiguous expression or non-description even in current textbooks, original CG graphics by quantum chemistry calculation are also provided for students (Figure 6). Students come to understand the coordinate steadily at the time of the corresponding experiment.
The current situation of teacher education in Japan and the development of the training program with experiment study were introduced. Beginning in March 2009, MEXT (the Ministry of Education, Culture, Sports, Science and Technology) has enforced a system of "certificate renewal course" that requires educators to acquire the most advanced knowledge and skills every 10 years in cooperation with a university. MEXT has also begun a comprehensive review of policies to improve the quality of teachers, including enhancement of pre-service teacher training courses in universities. The board of education in each jurisdiction prefecture also has enforced originally in accordance with the guidelines for elementary and secondary education. The challenges as Tokyo Gakugei University and as the department of chemistry to pre- and/or in-service teacher training were introduced. The project learning programs and the experiment learning programs have been developed.
One of the goals of teaching science should teach the process of science [6] or give students the opportunity to learn or appreciate the process. The 1996 NSES expects teachers to plan and incorporate inquiry into the science curriculum [7]. Some of the student outcomes listed in the NSES document include the ability to design and conduct scientific investigations, formulate scientific explanations using experimental evidence, and effectively communicate the results of scientific investigations. Research has shown that students using a laboratory-investigative approach show significant gains in formulating hypotheses, making assumptions, designing and executing investigations, understanding variables, making careful observations, recording data, analyzing and interpreting results, and synthesizing new knowledge, as well as the development of curiosity, openness, responsibility, and satisfaction [8]. Research usually needs patience, and this is one of the important factors in science research. Teachers' stance, how teachers can successfully incorporate inquiry into the laboratory without overwhelming themselves or the students [9], is needless to say also important.
Thinking and behaving imaginatively in science would be important
to promote creativity as outcome with value to the original objective
[10-13]. Child and/or Osborne, et
al. mentioned that students should appreciate that science
is an activity that involves creativity and imagination as much
as many other human activities, and that some scientific ideas
are enormous intellectual achievements [14,15].
Creative thinking is a key for students to have images of objectives
as in phenomena, chemical concepts, and molecular world in chemistry
through inquiry-based learning as like problem-solving, subject
research, etc. [16]. It is important for student to have
thinking and behaving imaginatively, and finally to have an outcome
which is of value to the original objective [10,11].
Promoting creativity in science has been reported and discussed
in papers [14,15,17-19]. Scientists, as much as any other
profession, are passionate and involved humans whose work relies
on inspiration and imagination as mentioned by Osborne [15].
Even in science education, it is more desirable that students
should educate themselves in a similar manner. Challenges of inquiry-based
instruction in Japan have been advocated so far by the Ministry
of Education, Culture, Sports, Science and Technology (MEXT) with
the guidelines of the Courses of Study [20].
The subject of "Science Subject Research" was newly
established for the purpose of the cultivation of a base of students'
creativity through the inquiry-based learning in high school-level
science [21]. Learning through experiment
study on the basis of students' enthusiastic activities with imaginative
thinking and behaving would be of great importance to understand
science. Realizing images led to understanding are expected to
be enhanced students' enthusiastic activities. Student's attitude
being enthusiastic toward the possibilities of their own abilities
with their own images would enhance the understanding of objectives
besides with the hope of acquiring sufficient knowledge and skills
through experiment learning program. Further development of the
research on this field is sincerely expected.