Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-107/Received August 31, 2013.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
The purpose of this study was to identify the difference of
learning performance on physical science concepts from high and
low achievement students after traditional instruction. The subjects
of this study were 100 nursing students coming from two classes
of a medical care junior college in north Taiwan. This study was
conducted in the first term of academic year 2011. One-group pretest-posttest
design was adopted. All students were assigned to high and low
achievement groups by item analysis of pretest scores, and research
tool is physical science test. The result found that high achievement
students performed better than low achievement students among
pretest, midterm, and posttest. During the learning process, high
achievement students' midterm scores performed lower than pretest;
in addition, posttest scores performed better than pretest and
midterm. Instead, low achievement students' scores gradually improved
during learning period. No significant difference was found on
both groups' midterm and posttest scores. High achievement students'
pretest scores were significantly better than midterm, and posttest
scores were significantly better than pretest. In contrast, low
achievement students' posttest scores were significantly better
than pretest. There were significant differences in scores on
"electronic configuration (item 3)", "electronic
energy levels (item 6)", " the comparison of gravitational
force and electromagnetic force (item 10)", and "whether
magnetic and electrics forces are related? (item 11)" concepts.
However, low achievement group has significant improvement in
physical science concepts. We suggest that teachers could infuse
films or animation and increase students' active learning activities
in chemistry class to enhance students' understanding of these
abstract concepts. Results of this study can be the ground of
improving chemistry teaching, and ultimately achieving the goal
of citizen scientific literacy.
Keywords: comparison of high and low achievement groups, learning performance, physical science concept of junior college students, comparison of pretest midtest and posttest achievements
Contents
Motivation
Chemical and physical sciences is closely bound up, and they
are the most important basic knowledge regarding to science education.
Because students' extent of physical science concept will affect
their learning performance, consequently recognizing students'
concept understanding of physical sciences become one of the most
important topics to chemistry education.
Traditional teaching is often viewed as rigid and insufficient
for attracting students' learning interest. Hence in recent years,
many scholars have tried to improve students' science learning
effect by a variety of teaching methods or assessment tools (Holbrook & Rannikmae, 2007; Lee, & Yi, 2013; Laugksch
& Spargo, 1996; Spektor-Levy,
Eylon , & Scherz, 2009).
However, traditional teaching method is still the main way in
large classes currently. Rarely research explores different-achievement-students'
learning process and the learning performance under this method.
Further, in Taiwan, to implement the twelve-year public education
program, multiple entrance system for junior high school students
makes them easily available to acquire entry qualification. Subsequently,
the gap of students' basic scientific competency gradually increased.
The base of the understanding of scientific concepts is the groundwork
to foster scientific literacy, hence probe students' learning
process can facilitate teachers recognizing students' learning
weaknesses. The purpose of this study was to establish the basis
of research declaration above, and finally proposes suggestions
for teaching improvement.
Research questions
(1) Is there any performance difference between high and low
achievement group students' in chemistry learning process?
(2) Is there any difference on physical science concept between
high and low achievement group students?
Literature Review
Bridle and Yezierski (2011) argued
that "students in traditional college-preparatory chemistry
courses become masters of mathematical equations without an understanding
of the conceptual basis for the mathematical relationships".
For this purpose, this study focuses on understanding physical
science concepts, because these concepts are difficult for students,
especially in the upper secondary schools or junior college stages.
Physical science concepts include forces, gravity, light, waves,
energy, electronic configuration, physical change, and chemical
change (Stein, Larrabee, & Barman,
2008). Mounting evidence shows that it is difficult for students
to understand how this world operate by their viewpoint toward
force and motion (American Association
for the Advancement of Science, 1993; Watts
& Zylbersztajn, 1981). For instance, children are difficult
to understand lunar gravity, and the Earth gravity on different
heights as well as gravity of objects at rest (Watts
& Zylbersztajn, 1981).
Halloun and Hestenes (1985) indicated
that college students are not only in short of basic physical
concepts, but also are firmly in misconceptions in place. Also
Zeilik, Schau & Mattern (1998)
asserted that college students are more difficult to change their
physical science concepts than to do in astronomy.
Johnstone (1982) proposed that chemical
knowledge can be represented in three main ways, such as macro,
sub-micro and representational knowledge. Macro indicates the
level of entity, which can be touched, seen, and can be used.
Micro means the level of molecular, structure and bonding. Representations
are referred to as the level of symbols, equations and calculation.
Atomic structure and its related concepts are microscope of natural
phenomena, especially the particle nature of matter is very important
in the chemical learning (Yezierski &
Birk, 2006), they deeply affect science and technology development
(Nicoll, 2001; Özmen,
2004; Tan & Treagust, 1999;
Ünal, Co_tu, Ayas, 2010).
Similarly, a particulate understanding of atoms and their properties
is central to explaining any chemistry concepts (Gabel,
1999). But Novick and Nussbaum (1978)
concluded that Grade 7 students are difficult to assimilate particle
model; moreover, most of them with their sensory perception of
matter are inconsistent.
In the past 30 years, there have been a large number of results
in physical science research; however, the broader use of assessment
tools are not much, some of them focus on one topic such as physical
or chemical changes, while others are large-scale investigated
by scientific literacy test. As "The Science Belief Test"
developed by Stein, Barman and Larrabee, an online assessment
tool which contains 47 true false declarative items, each question
accompany writing explaining to confirm the common beliefs and
alternative conceptions of students (Stein,
et al., 2008). Additionally, Laugksch and Spargo (1996) also
developed a 110 items assessment tool -Test of Basic Scientific
Literacy (TBSL). The tool contains physical science section, and
the assessment subject from high school students to citizens.
The scope of the study pervades United States, Africa, Hong Kong,
China and Taiwan.
Science literacy is the important goals in contemporary science
education (Brown, Reveles, & Kelly,
2005; Holbrook & Rannikmae, 2007),
because the science is one of the greatest achievements in human
cultures until now, moreover affects our lives. This study employed
physical science test which was extracted from TBSL as target
tool. Since the physical science is the basis for chemistry learning,
this study was to analyze academic performance of different achievement
students in the physical scientific concepts.
Methodology
Participants and procedure
Purposive sampling was employed in this study and the sample
consists of 100 freshmen enrolled department of nursing; the students
are from the junior college that is located in northern Taiwan.
This study was implemented in the first term of academic year
2011.
This study adopted one-group pretest-posttest experimental method,
chemistry course was scheduled as two hours per week and the total
contact hours were 24. There are four control variables as follows.
(1) Materials: all students accepted the same materials (introduction,
material science, atomic structure and periodic table, chemical
bonding and other four chapters).
(2) Background factors: all students were freshmen nursing students,
most of whom were female.
(3) Teaching period and examinations: 13 weeks, including pretest
(first week), midterm (ninth week) and posttest (thirteenth week).
(4) Instructor: the same person (researcher).
Participant's pretest score and item analysis was conducted to
distinguish students' achievement level. Top 27% of pretest scores
were classified as high achievement group, the last 27% of pretest
scores was classified as low achievement group.
Tools-Physical Science Test (PST)
PST was from physical science of science content category
in TBSL (Laugksch & Spargo, 1996)
and was revised by 2 different chemistry teachers. The goal of
TBSL is to examine the success of scientific literacy for school;
the results can provide teachers to reflect on how to improve
science teaching. Most of the questions were learned in junior
high school, and the test level is coping with students' ability.
The concept includes forces, gravity, light, wave energy, electron
configuration, Physical change, and chemical changes. There are
14 original items; further 3 items were deleted by using the value
of the calculated critical ratio. Final test contains 11 true
false items (Appendix 1).
70 freshmen were randomly select from other classes and agreed
to examine PST. The reliability of PST was calculated .624 by
KR-20 method.
Data collection and analysis
Three paper-and-pencil tests, such as pretest, midterm, and
posttest were administered to both high and low groups at three
stages of the study to assess students' understanding of physical
science concepts during the learning process. The maximum scores
for three tests were of 11 marks.
Data obtained were analyzed by using quantitative data analysis
techniques. The statistic methods include descriptive statistics,
pair t-test and covariance analyses.
Result
1. Descriptive statistic
There are 26 participants from high and low achievement group,
respectively. High achievement group's midterm scores were lower
than pretest, and posttest scores were higher than pretest. Low
achievement group has gradually improved their scores during learning
process. Descriptive statistics about both groups' performance
is shown in Table 1. The performance
of both groups is shown in Figure 1.
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
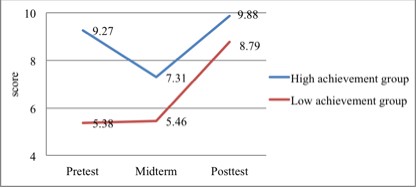
2. Compare both groups' performance in learning process
Analysis of covariance was employed to examine both group's
performance difference in learning process. Result showed that
when pretest total scores were controlled, the difference between
groups was not significant with respect to midterm adjusted mean
scores [F(1-49) = 2.60, p>.05]. Although a significant difference
did not exist between the midterm mean scores of the groups, the
mean of the high achievement group (X = 7.31) was higher
than that of the low achievement group (X = 5.46).
Result showed that when pretest total scores were controlled,
it was not significant with respect to posttest adjusted mean
scores [F(1-45) = .04, p>.05]. Although a significant difference
did not exist between the posttest mean scores of the groups,
the mean of the high achievement group (X = 9.88) was higher
than that of the low achievement group (X = 8.79).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
3. Performance of high achievement group in learning process
Pair-t test was employed to examine high achievement
group's performance in learning process. The results shows that
item 3, 6, 10, 11 and total scores presented significant differences
in pretest and midterm (Table 4),
and pretest score is higher than midterm score. In the aspect
of pretest-posttest, only total score presented significant differences
and posttest scores is higher than pretest (Table
5).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4. Performance of low achievement group in learning process
Pair-t test was employed to examine low achievement
group's performance in learning process. The results show that
no significant difference was found in pretest and midterm (Table 6). In the aspect of pretest-posttest,
items 1, 3, 4, 6, 7, 8, 10 and total scores presented significant
differences, and posttest score is higher than pretest (Table
7).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Discussion
Learning performance of physical science concepts between high
and low achievement students
This study reveals that midterm scores (at ninth week) of
high achievement students' physical science concepts have significantly
lower than pretest scores at "electron configuration"
(item 3), "electronic energy levels" (item 6), "the
comparison of gravitational force and electromagnetic force "
(item 10), "whether magnetic and electrics forces are related?"
(item 11) and total score (Table 4, 5).
In the thirteenth weeks, posttest scores of high achievement group
were significantly better than pretest scores. Results shows that
high achievement students' errors concepts aroused at ninth week
and it will be significantly improved until the thirteenth week.
Based on the book of "Benchmarks for Science Literacy"
(AAAS, 1993), high achievement students'
error concepts can be classified to science content as follows:
(1) force of nature (item 10, 11), (2) structure of matter (item
3), and (3) energy transformations (item 6).
In the aspect of force of nature, gravitational force is an attraction
between mass. Hence, the gravitational forces between atoms are
quite weak because of their masses are completely in light weight.
Besides, the strength of the forces is affected by their distance.
The longer the distance, the weaker the force is. These are the
nature of microscopic phenomenon.
Electromagnetic forces acting within and between atoms are vastly
stronger than the gravitational forces acting between the atoms
(AAAS, 1993). The positive and negative
charge atoms make the molecule as a whole electrically neutral.
High achievement students had difficulty in distincting the gravitational
force and electromagnetic force. Our findings are similar to the
statement of Watts and Zylbersztajn (1981), who presented that
students confused the earth gravity on different heights. It implies
that teachers could list their difference and explicitly express
the definition of these two science concepts to eliminate errors
in students' ideas.
In addition, magnetic forces are very closely related to electric
forces and can be thought of as different aspects of a single
electromagnetic force (AAAS, 1993).
This is because moving electric charges produce magnetic forces
and moving magnets produce electric forces. Most of modern technologies
work by interaction with electric and magnetic forces, and produce
the electromagnetic waves.
A paradoxical idea for students is how weak gravity is compared
to electric and magnetic forces (AAAS, 1993). For students, gravitational
forces seem stronger than trivial electric forces, e.g.
combing the dry hair. However, it seems that students could hardly
recognize that small amount of charge could force the dry hair
up against gravity.
Study of the nature of electric and magnetic forces should be
joined to the study of the atoms (AAAS, 1993). The atomic theory
can powerfully explain many phenomena, but it demands imagination
and evidence inference, as the results of electric forces and
magnetic forces are invisible. The priority should be put on what
conditions produce a magnetic field and what conditions induce
an electric current.
In the aspect of structure of matter and energy transformations,
energy levels are associated with different configuration of atoms
and molecules (AAAS, 1993). Besides, it is difficult to understand
which other features of the reactions between iron and chlorine,
or hydrogen and oxygen, for students are expected to deduce from
atomic electronic arrangements (Taber,
2003). Therefore, teacher should emphasize the importance
of electronic configuration and energy levels of atoms in chemical
reaction.
As mentioned above, error concepts may be due to mutual influence
of new knowledge and old experiences, resulting in midterm scores
lower than pretest scores. However, with the increase of teaching
content, student's physical science concepts will gradually meet
the scientific view.
In the thirteenth week, more than half of the questions (items
3, 4, 6, 7, 8, and 10) and the total score of low achievement
students' performance were significantly better than pretest scores
(Tables 6 and 7). These results show
that their physical science concepts will be significantly improved
at least last 13 weeks learning. The main cause might be their
incomplete or insufficient prior knowledge or proficiency of information
process.
According to the cognitive development theory, Piaget
(1964) believed that students have been formal operational
stage between the ages of 14-15 year-old, and they should be able
to understand abstract concepts. However, the results of this
study show that, regardless of the level of students (high or
low achievement), they must undergo 13 weeks of learning to achieve
significant improvement; in other words, after a period of time,
traditional teaching may facilitate students' conceptual understanding
about abstract concepts in chemistry.
Limitations of the study
Due to the gender unequality (most of participants are female
in nursing classes), this study's findings may not infer to other
learning set. Besides, as the sample size of the study was small,
it needs to be supported by larger-scale studies to reveal the
effects of traditional instruction. The average of students' entrance
PR values was 30, where the PR value indicates the student's academic
achievement surpassing other students' in number, and ranging
from 0 to 100 (the higher the value of a student, the better his/her
academic achievement is). Thus, their academic achievement levels
were below 50% of the same grade in Taiwan. At least under these
conditions, students' learning performance may be different from
other students with different PR values.
Conclusion
Citizens' scientific literacy has become one of the most important
goals toward science education in many countries. Understanding
the difference in physical science concepts learning acquired
between high and low achievement students is helpful for further
enhancement of citizens' scientific literacy. This study provides
an empirical research example and results, and the conclusions
are as follows:
(1) There was no significant difference on learning performance
between high and low achievement groups.
(2) Pretest scores of the high achievement group were significantly
better than midterm, and posttest scores were significantly better
than the pretest; the posttest scores of low achievement group
were significantly better than the pretest.
(3) In the ninth week, high achievement group students have significant
errors in concepts on "electronic configuration", "electronic
energy levels", " the comparison of gravitational force
and electromagnetic force ", and "whether magnetic and
electrics forces are related?". But posttest scores (thirteenth
week) of both the high and low achievement groups were significantly
better than pretest.
Suggestion
(1) Different energy levels are associated with different
configurations of atoms and molecules (AAAS,
1993). High achievement group students have errors in the
concept of energy levels and electronics configuration in the
midterm. Results might be due to poor visualization capacity (Gabel, Samuel, & Hunn, 1987). This
study suggests that teachers may infuse films or animation in
chemistry class to enhance students' understanding of these abstract
concepts.
(2) According to the opinions of cognitive learning theory, Novak and Gowin (1984) argued that teachers'
task is to try to find ways to increase meaningful learning, possibly
by actively involving students in the process of knowledge construction.
Consequently, it is recommended that teachers could increase students'
active learning activities, such as ask questions, discuss, or
conceptual understanding strategies to strengthen students' meaningful
learning, ultimately to enhance students' understanding of physical
science concepts, and even shorten the learning time.
Reference
American
Association for the Advancement of Science (1993). "Benchmarks
for Science Literacy". Oxford University Press, New York.
Bridle, C. A., & Yezierski, E.
J. (2011). "Evidence for the effectiveness of inquiry-based,
particulate-level instruction on conceptions of the particulate
nature of matter", Journal of Chemical Education,
89 (2), 192-198.
Brown, B. A.; Reveles, J. M. &
Kelly, G. J. (2005). "Scientific literacy and discursive
identity: A theoretical framework for understanding science learning",
Science Education, 89, 779-802.
Gabel, D. L.; Samuel, K. V. &
Hunn, D. (1987). "Understanding the particulate nature of
matter", Journal of Chemical Education, 64
(8), 695-697.
Gabel. D. (1999). "Improving
Teaching and Learning through Chemistry Education Research: A
Look to the Future", Journal of Chemical Education, 76
(4), 548-554.
Halloun, I. A. & Hestenes, D.
(1985). "The initial knowledge state of college physics students",
American journal of Physics, 53 (11), 1043-1055.
Holbrook,
J. & Rannikmae, M. (2007). "The Nature of Science Education
for Enhancing Scientific Literacy", International Journal
of Science Education, 29 (11), 1347-1362.
Johnstone, A. H. (1982). "Macro
- and micro - chemistry", School Science Review, 64,
377-379.
Korpan, C. A.; Bisanz, G. L.; Bisanz, J. & Henderson, J. M.
(1997). "Assessing Literacy in Science: Evaluation of Scientific
News Briefs", Science Education, 81, 515-532.
Laugksch,
R. C. & Spargo, P. E. (1996). "Construction of a paper-and-pencil
test of basic scientific literacy based on selected literacy goals
recommended by the American Association for the Advancement of
Science", Public Understanding of Science, 5,
331-359.
Laugksch, R. C. (2000). "Scientific literacy: A conceptual
overview", Science Education, 84 (1), 71-94.
Lee, G. & Yi, J. (2013). "Where
Cognitive Conflict Arises From?: the Structure of Creating Cognitive
Conflict", International Journal of Science and Mathematics
Education, 11 (3),601-603.
Nicoll, G. (2001). "A report
of undergraduates' bonding misconceptions", International
Journal of Science Education, 23, 707-730.
Novak, J. D. & Gowin, D. B. (1984).
"Learning How to Learn", Cambridge University Press,
Cambridge.
Novick, S. & Nussbaum, J. (1978).
"Junior high school pupils' understanding of the particulate
nature of matter: An interview study", Science Education,
62 (3), 273-281.
Özmen, H. (2004). "Some
Student Misconceptions in Chemistry: A Literature Review of Chemical
Bonding", Journal of Science Education and Technology,
13 (2), 147-159.
Piaget, J. (1964). "Part I: Cognitive
development in children: Piaget development and learning",
Journal of research in science teaching, 2 (3),
176-186.
Spektor-Levy, O.; Eylon, B-S. &
Scherz, Z. (2009). "Teaching Scientific Communication Skills
In Science Studies: Does It Make A Difference?", International
Journal of Science and Mathematics Education, 7, 875-903.
Stein,
M., Larrabee, T. G., & Barman, C. R. (2008). "A Study
of Common Beliefs and Misconceptions in Physical Science",
Journal of Elementary Science Education, 20 (2),
1-11.
Taber, K. S. (2003). "The atom
in the chemistry curriculum: Fundamental concept, teaching model
or epistemological obstacle?", Foundations of Chemistry,
5 (1), 43-84.
Tan, K. C. & Treagust, D. (1999).
"Evaluating students' understanding of chemical bonding",
School Science Review, 81, 75-84.
Ünal, S.; Co_tu, B. & Ayas,
A. (2010). "Secondary School Students' Misconceptions of
Covalent Bonding", Journal of Turkish Science Education,
7 (2), 3-29.
Watts,
D. M. & Zylbersztajn, A. (1981). "A survey of some children's
ideas about force", Physics Education, 16 (6),
360-365.
Yezierski, E. J. & Birk, J. P.
(2006). "Misconceptions about the Particulate Nature of Matter
Using Animations to Close the Gender Gap", Journal of
Chemical Education, 83, 954-960.
Zeilik, M.; Schau, C. & Mattern,
N. (1998). "Misconceptions and their change in university-level
astronomy courses", The Physics Teacher, 36,
104-107.
Appendix
1: Physical Science Test
1. Everything is made of over one hundred chemical elements and
formed by different combinations in the material world.
2. Each material may exist in different states (e.g., solid, liquid
or gaseous) by different temperature and pressure.
3. Atomic bonding between atoms is determined by outer layer electron
arrangement of each atom.
4. When a certain state of energy (for example heat) or some places
of energy reduce, another state or place of energy will equally
increase.
*5. Atomic arrangement in the molecules has nothing to do with
the energy of molecules.
6. Electron energy levels are not continuous.
7. Nothing is stable among atoms, organisms and planets, and all
of them are always activated.
8. Motion is caused by imbalance forces.
9. Every object will create gravitational force on other objects
in the universe.
10. Electromagnetic force is larger than gravitational force when
it acts on the atoms.
*11. Magnetism and electricity force are unrelated to each other.
(* indicates wrong answer)