Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-108/Received August 31, 2013.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
Airborne particles have been collected in Gifu located in
a middle of the Main Island of Japan. The particles were observed
by scanning electron microscopy (SEM), analyzed by energy dispersive
X-ray spectrometry (EDX), and classified into soil, pollen, sea
salt, soot and some cosmic dust. We had made the website containing
the SEM photos of these particles and the movements of soil particles
related with Kosa (yellow sand) and of sea salt crystals in the
atmosphere related with the weather, aiming at offering the teaching
and learning materials to teachers and students in primary and
junior-high schools [1]. Students
learn the global circulation of water in the atmosphere in connection
with the climate in the second grade of three-year junior high
school, after they learn three states of water in the fourth grade
of six-year primary school and in the first grade of three-year
junior high school. This time we added dynamic images of snow-like
ice crystal growing to our website and reconstructed the whole
site. The website was renewed using Cascading style sheets (CSS)
in addition to HTML files consisting of SEM photos and EDX spectra
of airborne particles. CSS controls basic designing of all the
pages, simplified the programs in HTML files, and decreased capacities
of the files. Each pair of SEM photos and EDX spectra of these
particles was allocated to a different page of the site. Then
the whole site became improved with shorter access time and clearer
photos of particles. Through these airborne particles and ice
growing, we expect students to realize wonders in our daily life
and connection from our close surroundings to huge surroundings
of the earth.
Keywords: web-based material, teaching material,
airborne particles, ice crystal growing, science education, environmental
education, atmospheric movement
Reconstruction and addition of web pages for ice crystal growing
The courses of study for primary and junior high schools were revised in 2008, and improved courses have started in 2009 with renewed textbooks. Before the revision the textbooks for junior high schools were divided into two parts; the first one consists of physics and chemistry fields, and the second biology and earth science fields. That time, it was a little difficult for students to connect the changes of state of water in the first part to the circulation of water in the atmosphere connecting with weather in the second part. These have been rearranged to three textbooks for three years of junior high schools, each of which contain all the fields. After this revision, it seems to become easier not only for students but also for us to study science more continuously and to develop interdisciplinary teaching materials more smoothly to connect science to environmental education.
When we look at the contents of textbooks in primary and junior high schools, however, we still notice that in the chemistry field we mainly deal with substances and phenomena in a lab, that is, indoor science, using test tubes, beakers, and some other experimental apparatus. This gives the students the impression that chemistry is a field which is done only in a lab and in which we synthesize something artificial. Senior students know that chemistry has made up so many industrial materials which have been contributing to our civilized and convenient life very much. But they also realize the other side of chemistry making harmful substances causing problems such as pollution. All these words, convenience, civilization, and pollution seem to be connected to chemistry with a keyword of artificial. The word "artificial" is opposed to "natural". Then for students chemistry seems to be far away from nature. We wish to break such images of chemistry.
We have been observing airborne particles and seeing natural atmospheric behavior. In junior high schools, students learn the properties of gases and solutions and the three states of matter. They also learn weather, atmospheric movement, and minerals. These are parts of earth science, but we would like to look at those from chemistry side through the airborne particle observation. Also we would like to extend the students' understanding about chemistry to the geochemical movement of the earth itself.
We add pages for snow-like ice-crystal growing. Students can get ice easily in a refrigerator at home. Snow is also ordinary stuff in daily life in Japan in winter. They are not so mysterious or exciting things for them. However, if they look at its growing process, they would be aware of wonder in nature. Since we could not observe real snow growing, we tried to grow snow-like ice crystals in our lab by using cooling apparatus.
From these materials, we expect students to extend their sight from their close surroundings to the global atmosphere and at the same time to make them realize the relation between the material world and the environment for living things. This is an important role especially for the chemical education that introduces students to the material world.
Airborne particle observation
Airborne particles have been collected and observed in the
same way as in our previous work [1]
by using a scanning electron microscope, SEM (Hitachi S-4300)
and an energy dispersive X-ray spectrometer, EDX (Horiba EMAX)
operated in Instrumental Analysis division, Life Science Research
Center, Gifu University.
Ice crystal growing
The procedure for ice crystal growing was reported in our
previous work [2]. However, since
it was written in Japanese, we explain it here again.
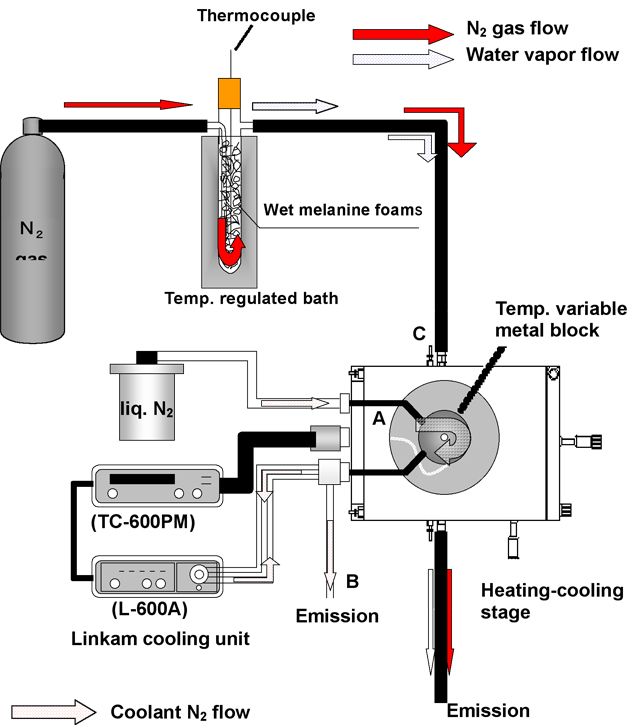
Figure 1 shows the schematic drawing of the apparatus for ice crystal growing. The temperature of the glass container having wet melanine foams was set at 0 °C (between 0 and -2 °C) and we show this temperature as Tinput. The N2 gas flow from a cylinder was regulated between 1 and 3 L/min with a gas flow gauge. Water vapor evaporated from water or ice in many small pieces of melanine foams was sent to the cooling-heating stage with N2 gas flow. Ice crystals were grown at various temperatures of the metal block from -7 to -30 °C controlled with Linkam TC-600PM and L-600A cooling unit. The dynamic images were observed and recorded with motion analyzing microscope (Keyence VW-5000) and wide range zoom lens (Keyence VH-Z100). The temperature of the surface of the glass plate on the metal block was measured from -5 to -255 °C respectively with a copper-constantan thermocouple at the same conditions as the crystal growth independently from the observation.
Reconstruction and addition
of web pages for ice crystal growing
Reconstruction of our web-based material about airborne particles
[3] was reported previously [4].
Figure 2 shows the top page of our
website to get in the pages about airborne particles and snow-like
ice crystal growing [5].
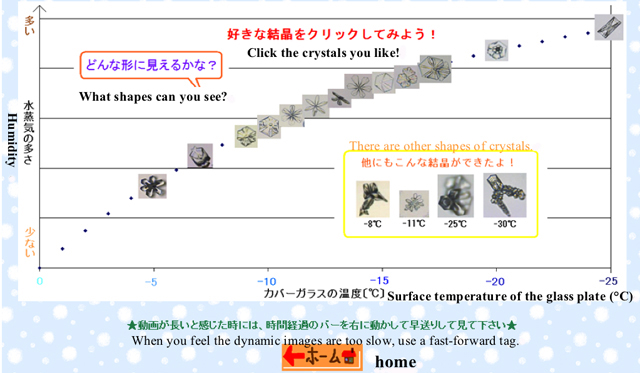
Figure 3 is a page showing the relation between the forms of crystals and the conditions of temperature and humidity where ice crystals were grown. As shown in our previous report [2], the excess vapor density (Δρ(gm-3)) under supersaturations is
where Δp is the difference between saturated vapor pressures at Tinput and at the surface temperature (T) of the glass plate, and 18 is a molar mass of water. varies along with the dotted line shown in Figure 3. When we click each of photos of these crystals in Figure 3, we can see dynamic images of the crystal growing process, which was edited by DVgate Plus.
We mentioned several sample fragments to use these teaching materials about airborne particles previously[1]. The EDX spectra of those particles show that all the natural things found in the atmosphere consist of chemical elements. These several years, the Ministry of Education, Culture, Sports, Science & Technology in Japan has been promoting understanding of chemical elements through distribution of periodic tables of the elements to all the students' houses, in which major products made from the respective elements were exemplified. The EDX spectra can help students to understand where some of the elements come from.
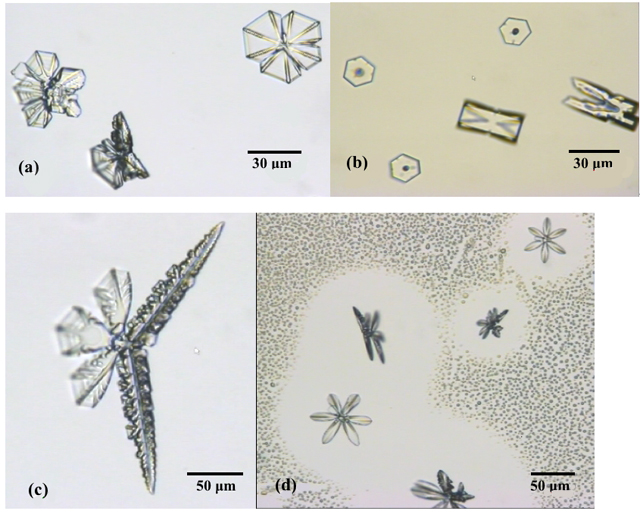
This time we added snow-like ice crystals grown from water vapor.
Although we show the various shapes of ice crystals in
Figure 3, some of real snow crystals
in nature have more dendritic forms. Therefore students might
be disappointed in dynamic images of ice crystal growing here.
We have been trying to find the conditions under which more dendritic
crystals are grown and now we have recorded the growing process
of more dendritic ice crystals. We will add it soon. However,
we expect the present ice crystal growing process makes students
understand that this is not the solidification from liquid to
solid but the 'desublimation' process from gas to solid and that
invisible water molecules are supplied to ice crystals to grow.
We also expect these make them imagine dynamic behavior in the
atmosphere easily. Some of crystals we grew and the growing shot
in which we can understand 'desublimation' are shown in Figure
4.
Some lessons using these materials are in progress now by collaborating primary school and junior high school teachers [6].
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 25350196.