mail: ikuo
Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-109/Received August 31, 2013.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
CG teaching material of hydroxylation of chloromethane as
a model of Walden's inversion was produced. The teaching materials
could demonstrate the nature of the reaction such as structural
change by ball-and-stick model or space filling model, and potential
energy change by the reaction profile. From the survey, students
were able to obtain the image of drastic change in the structure
on the inversion from the teaching material. Electric textbook
for chemical laboratory, which integrates observable level experiment
and molecular level teaching material, is trying to produce.
Keywords: CG, Teaching Material, Electronic Textbook,
Walden's Inversion
Chemical education has the circumstances performed through an experiment. Understanding the observed phenomena, chemists use to imagine and explain observations in terms of molecules. Observed phenomena and molecular level models are then represented in terms of mathematics and chemical equation [1, 2]. Student's difficulties and misconceptions in chemistry are from inadequate or inaccurate models at the molecular level [3]. Visualization is great help for students to have images in the molecular level. It is our aim to produce computer graphics (CG) teaching material based on quantum chemical calculations, which provides realizable images of the nature of chemical reaction [4]. If the CG teaching material is combined with chemical experiments of student's laboratory, students would observe the reaction from three thinking levels, namely, phenomena in the observable level and CG teaching material in the molecular level, and chemical equation in the symbolic level. Our ultimate goal is to produce such an electronic textbook that can be used not only in the classroom but also in the experimental laboratory.
Walden's inversion is one of important example of the bimolecular nucleophilic substitution reaction (SN2 reaction), which inverse configuration of reactant. Therefore, the reaction is often adopted in teaching material on the curriculum of the university, including some appropriate schemes [5]. The schemes should be developed for student to acquire more realizable images of the nature of the reaction. We developed CG teaching material for university student, concerned about reaction with drastic change of the structure of reactants in the reaction (Eq. 1) as a model of Walden's inversion.
This paper describes an approach to the electronic textbook of basic chemistry linking chemical experiments, which integrates the observable level experiment and the molecular level.
The semi-empirical molecular orbital calculation software MOPAC [6] with PM5 Hamiltonian in CAChe Work System for Windows (Former name of Scigress, ver. 6.01, FUJITSU, Inc.) was used in all of calculations for optimization of geometry, for search of potential energies of various geometries of intermediates, for search of transition state, and search of the reaction path from the reactants to the products via the transition state. The optimized structure of the transition state was verified by the observation of a single absorption peak in the imaginary number by the use of the program Force in MOPAC for vibration analysis. If the peak was observed, Intrinsic Reaction Coordinate (IRC) [7] calculation was done and the reaction path was confirmed.
A movie of the reaction path was produced by the software DIRECTOR (ver. 8.5.1J, Macromedia, Inc.) following the display of the bond order of the structure of the reactants in each reaction stage, which was drawn by the CAChe. It was confirmed that the drawn CGs of the molecular models of reactants moves smoothly. The red ball, which indicates progress of the reaction, was arranged on the reaction profile and simultaneous movements of the ball and the reactants were confirmed. The movie file was converted to the Quick Time movie by the Quick Time PRO (ver. 7.66, Apple, Inc.) and was saved to iPad (Apple, Inc.) by using the iTunes (ver. 10.7, Apple, Inc.).
Teaching material was practiced on three classes, the first year student of teacher training course for elementary school and the second year student of natural environmental science course, of "Chemistry laboratory" for the period of January 31 from January 25, 2011 at Tokyo Gakugei University. Total numbers of students were 103. All practice was carried out by one instructor and one teaching assistant. Teaching material used for the trial was the CG movie of the ball-and-stick model shown by the tablet PC because it was more effective to provide image than that with PC and the projector [8]. Procedure of the practice is described as follows. Preliminary survey, which consisted questions to ask them to draw image of Walden's inversion from formula (Appendix 1.1) and from scheme (Appendix 1.2), and questions about information of students, were conducted. Then, usage of the tablet was explained by instructor with projector. After the explanation, 9 tablet PCs were distributed to students and instructor asked them to watch CG movie in the tablet as shown in the Figure 1.

Students started to touch it and watched the movie over and over
enthusiastically. One tablet PC was shared by two to four students
depends on size of class. Time duration for usage of the tablet
PC was about three minutes for each student. During this time
period students were quiet which suggests that they were concentrated
on the subject. After about 10 minutes, students were asked to
start answer the posteriori survey that was to draw image of Walden's
inversion after watching the movie (Appendix 1.3). Some students
were still watching the movie even when they were answering the
questionnaire.
Reaction of hydroxide and chloromethane (Scheme 1) is a typical example of the Nucleophilic Substitution in the 2nd order reaction [5].
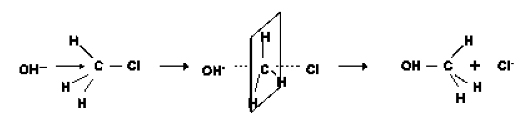
Carbon atom at center to which the halogen atom is attached is
attacked by the nucleophile, hydroxide, from a position away by
180 degrees and methyl alcohol forms. Therefore, the transition
state was searched from the reactants where the bond angle of
O-C-Cl was adjusted to 180 °.
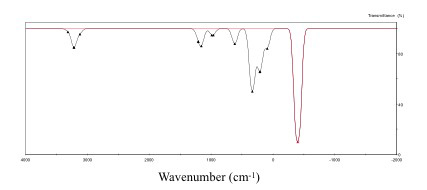
The result of the vibrational analysis is shown in Figure
2. A single absorption peak in the negative region was found
at -399.46 cm-1 from the vibrational analysis. This
result indicates vibrational mode due to the decrease of potential
energy for the direction of only one path via a true transition
state at the saddle point. The reaction path from the reactants
to the products via the transition state was searched by
the IRC calculation [7] in MOPAC [6].
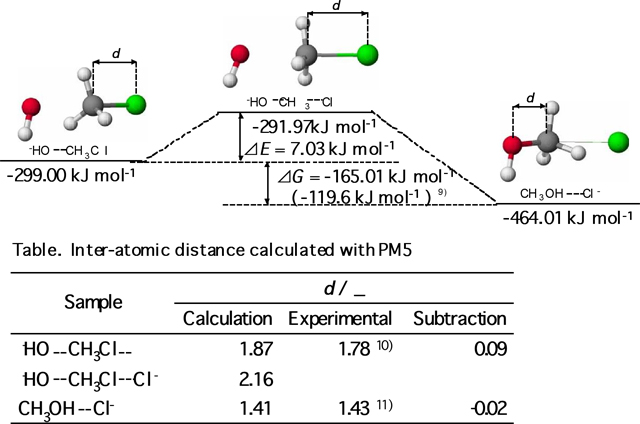
The inter-atomic distances, d, and the heat of formations
obtained by the calculations are listed in the scheme
2 and in the table therein. The inter-atomic distance of C-Cl
in CH3Cl was calculated as 1.87 Å (1.87 Å) [9],
and C-O in CH3OH was 1.41 Å (1.43
Å) [11]. Calculated values were
in good agreement with the literature values in the parentheses.
Energy difference between the initial state of reactants and the
final state of products was 165.01 kJmol-1. This value
was similar to the corresponding difference of literature values
(119.6 kJ mol-1) [9]. Therefore,
it was confirmed that the reaction path and the molecular configurations
obtained by the calculation were appropriate to produce CG teaching
material.
Selected pictures of CG teaching material are shown in the Figure 3. Structures were shown not only by the space filling model which shows realistic shape of the reactants but also by the ball-and-stick model which shows skeletal structure and bond strength. In the space filling, the existence probability of the electron is 90 %. In the ball-and-stick, the thickness of stick changes by bond order. Left part of the each model shows the reaction profile, potential energy vs. reaction coordinate, which indicates the degree of the reaction progress by the red ball on the profile. Right part shows structural change. A student is expected to obtain the image of an umbrella-reversal-like motion in Walden's inversion. When student touches the teaching material in the tablet computer, the Quick Time control bar appears and the red ball on the profile can move by student's choice. This manual control feature provides "Hands-on" feeling to student. This teaching material could provide not only images of energy change during reaction but also images of dynamic structure change during chemical reaction.
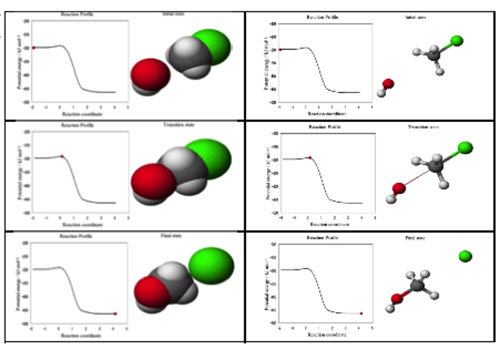
After tablet PCs were distributed to students they started to touch it and watched the movie over and over enthusiastically, which indicated that the teaching material created positive attitude among students. The practice was carried out within expected time schedule smoothly, which suggests that the usage of tablet PC in the laboratory class is suitable. If the laboratory class requires wet condition, such as synthesis, proper plastic bag type cover might be useful. From the result of the questionnaires, the answer judged to be able to acquire the image of structure change during Walden's inversion (the image of umbrella-reversal like motion) was follows; the image obtained from the CG teaching material (Appendix 1.3) was 51% while that from the reaction formula (Appendix 1.1) was 24%. The number in CG teaching material was better than that of the reaction formula. In the free description area (Appendix 1.3), some students described that movement of atoms during the reaction was easy to understand with CG teaching material (Appendix 2). Students were abele to obtain the image of drastic change of the structure in Walden's inversion from the CG teaching material easily. We are now trying to produce electric textbook for chemical laboratory (Figure 2), which integrates the observable level experiment and the molecular level.
In this work, the change in the molecular configuration in Walden's inversion made visible from the quantum chemistry calculation. The produced CG teaching material could provide images of not only energy change but also dynamical structure change during chemical reaction. From the practice, it was indicated that the usage of tablet PC in the laboratory class was suitable, created positive attitude among students, and was able to provide understandable image of drastic change of the structure in the Walden's inversion. Now we are trying to produce an electronic textbook in which the CG teaching material is combined with chemical experiments of student's laboratory.