Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-110/Received August 31, 2013.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
Since the 19th century, in United Kingdom there were some
evidences from the changes of crystal shape of a storm glass for
weather forecasts. However, literature indicated that the success
of prediction of the storm glass device was no better than random
probability. This report attempts to highlight the purpose of
evaluating the relationship between storm glass and weather with
solubility. Two experiments were conducted and data were recorded
and analyzed: the trend between the height of crystals and the
temperature of air and between the height of crystals and the
temperature of thermostatic water-bath. The results showed that
the height of crystals had a high degree correlation with the
temperature. The reason may be the solubility of camphor. On the
basis of the results, the storm glass device has a potential for
weather-thermometer.
Keywords: storm glass, crystal, camphor, solubility,
thermometer
Students often develop misconceptions about concept of solubility in solutions because students may not comprehend the relationships among saturation, temperature, and precipitation thoroughly [1-3]. By doing an experiment or operating a simulation associated with scientific history, students may learn concepts and to clarify the associated misconceptions [4, 5]. A "Storm Glass" is a type of device associated with solubility, a sealed glass jar filled with an ethanol solution mixed with distilled water, camphor, potassium nitrate and ammonium chloride [6-9]. Since the 19th century, in United Kingdom there were some evidences that storm glasses were used for the weather forecasts from the changes of the crystal shape in the device which was recorded by Robert Fitzroy who was captain of the Beagle, famous for Charles Darwin expedition [6-9]. Up until now, several factors affecting the storm glass have been examined and it has been found that only the temperature affect the crystalline state of storm glass [8]. It was the change rate of temperature that affected the crystal shapes and patterns, and in the detection of X-ray diffraction revealed that the crystal was camphor [6, 8]. Literature indicated that the success of the storm glass device in weather forecast was no better than random probability [6]. Previous studies have been focused on the components of the crystal and possible mechanism [7, 8]. However, there are only few reports to introduce solubility, rather than the crystal, of storm glass. The purpose of this study is to explore the relationship of the height of crystals of storm glass and temperature in the air or in the thermostatic water-bath.
Ethanol was HPLC grade reagents and d-camphor, ammonium chloride (NH4Cl) and potassium nitrate (KNO3) were of ACS grade. All materials were acquired from ECHO CHEMICAL. CO, LTD. in Taiwan, which is an agency of several famous chemicals companies.
The glass tubes [21 (diameter) X 200 (height) mm] were used to fill standard solution of storm glass. The thermostatic water-bath (DENG YNG Water Bath D-620) was used and the available temperature ranged from -20 to 100 °C (accuracy +/- 0.05 °C) electronically. The crystal ship was photographed by microscope (UNICO G395T-LED) with charge-coupled Device camera (magnification ratio 10 x 4.5).
The standard solution was prepared by mixing d-camphor (C10H16O, 30 g) with ethanol (100 ml). Another solution was prepared by mixing ammonium chloride (NH4Cl, 7.5 g), potassium nitrate (KNO3, 7.5 g), and distilled water to the total volume of 100 ml. Finally, the storm glass solution was made by mixing the two solutions at the same volume ratio.
Two experiments were conducted in order to examine the relationship between the height of crystal and the temperature. Owing to consider the needs in further applications in daily life, the the height of crystals in this study was measured visually. The three tubes filled with the standard solution of storm glass were plugged with cork. Preventing from ethanol evaporation storm glasses were sealed with parafilm outside the cork. The first experiment was that the tubes were placed outdoor next to windows. The temperature of the air and the height of crystals in the tubes were recorded for three months in Spring of Taipei. The average temperature of Spring in Taipei was about 18.9 °C last thirty years [10]. The second experiment of this study, the tubes above-mentioned were placed in a thermostatic water-bath with temperatures changing downward from 30 to 5 °C and upward from 5 to 30 °C with the rate of 1 °C per 15 minutes, and the height of crystals in the tubes was recorded.
The flammable solvent (ethanol) is used in the procedure. Gloves and goggles should be worn on measuring, handling, and mixing the reagents in a hood.
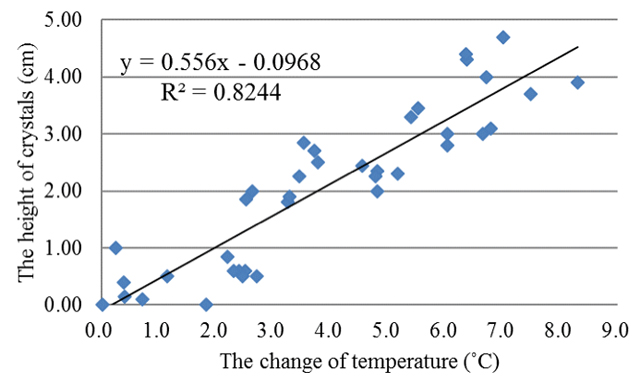
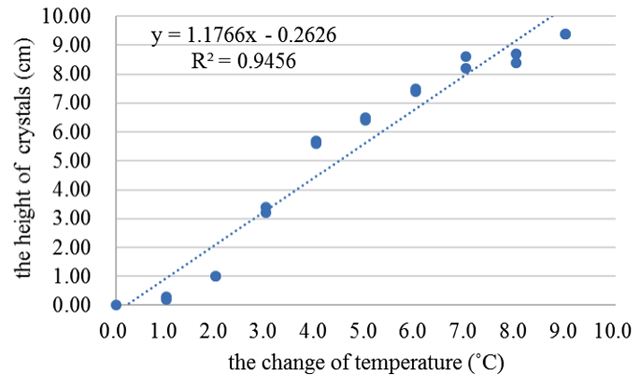
In fact, there were many factors such as temperature, humidity and pressure etc. that affected weather. Since the device of storm glass was plugged with cork and sealed with parafilm, it was only the temperature that environmentally affected the crystals or precipitation in the solution. The results of the first experiment was shown in Figure 1; there was a high degree correlation between the height of crystals and the change of temperature between the temperature of the air and all materials which were dissolved (ΔT1) in the storm glass (R2= .82). In Figure 2 was shown the relationship between the height of crystals and the change of temperature between the temperature of thermostatic water-bath and all materials which were dissolved (ΔT2) in the storm glass, and the variance was 0.95. The diagram revealed repeated cycles with downward and upward changes of 1 °C from 23 to 14 °C.
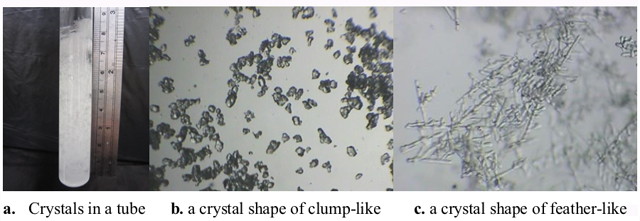
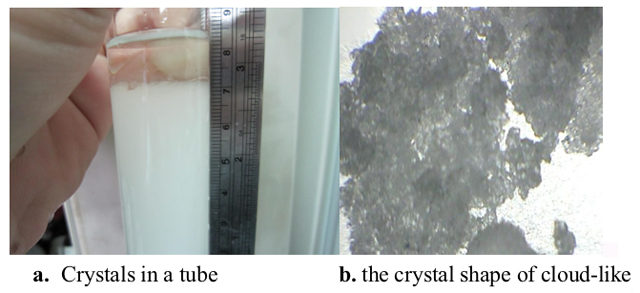
According to the detail records of Fitzroy, there were a relationship between weathers and the crystal shapes of storm glass [6-9]. Literature showed that temperature was the critical factor in using storm glass for weather forecasts [6]. As shown in Figure 3 and Figure 4, most of the crystal shapes (in the air and in the water-bath) were not similar to each other. It was probably that the thermal equilibrium was achieved in a shorter time in the water-bath than in the air, because of the higher rate of the heat transfer in the former case.
However, our purpose is to find out the relationship between storm glass and weather in terms of solubility, rather than to confirm the feasibility for weather forecasts based on the crystal shapes. On the basis of the results of this study, the storm glass has a potential tool for the thermometer in the 14 to 23 °C range. Above 23 °C, all the compounds were dissolved and below 14 °C, the solubility of the solutes in the standard solution was already so low that no appreciable amount of crystals were precipitated thereafter. The solubility curve of compounds does not show linear relationship with temperature [11], but it is still divided into several blocks each of which shows a particular relationship. In spite of the not-100%-linear relationship of the solubility curve, we can still observe the high degree relationship between height of crystals and the change of temperature. The size and diameter of the glass tubes in the experiments will affect the results of the height of crystals, but as the design of a thermometer, the thinner of the glass tube, the more sensitive it is.
Although the change rate of temperature affects the patterns of the crystal shape in the storm glass, previous studies do not indicate the relationship between the height of precipitation in the storm glass and the temperature of air or between the height of precipitation in the storm glass and the temperature of a thermostatic water-bath. Our results explain that solubility plays an important role in the storm glass and that the storm glass has a potential for a weather-thermometer. Authors will record for a much longer time to examine the relationship between crystal shapes and weather forecasts and try to innovate a weather-thermometer in the future work. High school students may learn scientific history of storm glass, practical usage of solubility and to clarify the relationship among saturation, temperature, and precipitation through these experiments.
The described interval of 15 minutes was determined from the
results of several preliminary tests. The changes of the height
of the crystals in the tubes were measured in intervals of the
temperature change (1 °C) including 15, 30, and 60 minutes.
Since there was no significant difference in heights of the crystals
in each temperature, the heights were measured in 15 minutes intervals
in the water-bath experiments.
Authors are grateful of appreciation for reviewers' comments.
This paper continues and expands the authors' conference (International
Conference of Network for Inter-Asian Chemistry Educators, NICE
2013) paper entitled Investigating the components of crystallization
and the relationship with weather in "Storm Glass".