Chemical Education Journal (CEJ), Vol. 15 /Registration No.
15-112/Received August 31, 2013.
URL =
http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
An experiment program titled "Determination of heat of
dissolution of NaCl" was developed for an undergraduate physical
chemistry laboratory class of the third year level students. The
program is composed of four parts: i) determination of heat of
the dissolution to water; ii) statistical data processing using
a spreadsheet-type program on personal computer; iii) calculation
of enthalpy change of the hydration; and iv) estimation of the
hydration number by entropy calculation.
Keywords: experiment program, physical chemistry laboratory,
heat of dissolution, NaCl
2 Class of physical chemistry experiment and its management
3 Development of experiment program
3.1 The target of an experiment
3.2 The outline of an experiment
Since the experiment about thermodynamics is important as the foundation of physical chemistry, the description can be found in the chemical experiment textbook of many universities [1]. Although many experiments deal with heat of the reaction, most of them remain the treatment of enthalpy change measured directly from an experiment, and we rarely see the contents about entropy. Born-Haber cycle of sodium chloride, NaCl, is a well-known procedure for student to learn thermochemistry. The measurement of heat of dissolution of NaCl, Hdissol, with value of 3.88 kJ mol-1 [2], is by no means an easy task for student in the laboratory class. Data logger type digital thermometers with a Pt-probe enable us to measure such a value. We developed an experiment program, "Determination of heat of dissolution", for an undergraduate physical chemistry laboratory class for the third year level students of a teacher's college, Tokyo Gakugei University. The program is composed of three parts: i) determination of heat of dissolution of NaCl to water (Hdissol = 4.48 kJ mol-1); ii) statistical processing of the data obtained in i) by means of a spreadsheet program on a personal computer, such as Microsoft Excel; and iii) calculation of the enthalpy change of hydration of NaCl from the Born-Haber cycle, and further, estimation of the hydration number of NaCl by entropy calculation.
Thirty-five science major students who belong to the chemistry department participated in the spring semester of 2012. They were from two divisions in faculty of education; a teacher preparation division: an elementary school education program and a secondary school education program, and liberal arts division: environmental comprehensive science program. The physical chemistry experiment includes three other themes such as; determination of Avogadro's number from the XRD and density measurement of NaCl, adsorption of benzoic acid on alumina, and measurement of the ESR signal of 2,2-diphenyl-1-picrylhydrazyl. Therefore, students were divided into four groups according to schedule of the themes. One group consists of three teams and one team consists of three students who conduct an experiment together and three teams are assigned to the same theme in parallel. The class uses two-hour period in a day and one theme is completed over two days in principle. Physical chemistry experiment is managed in a team teaching manner, which consists of three teachers and 2-3 teaching assistants.
The heat of dissolution is obtained by measuring the temperature change by the dissolution of sodium chloride in the water. The Born-Harbor cycle of sodium chloride is completed with the obtained value of the heat of dissolution and the values in literatures, and the enthalpy change of hydration is calculated. Furthermore, the hydration number of sodium chloride is estimated by calculating the entropy change of hydration. Through this simple experiment, mastering many things, such as understanding of thermodynamics, a computational technique, etc. is expected. It also aims at mastering the technique of an interpretation of thermodynamic data and statistical handling by using the physical parameter surveyed in this experiment.
Weekly schedule and the outline of concrete experiment are shown below.
Schedule of the experiment program
First week
Second week
The first week focuses on obtaining data that can be treated further. The second week is constituted so that students can concentrate on the statistical procedure and thermodynamic exercises of the data with a personal computer (PC).
Following seven assignments were given to the student.
1) Calculate the heat capacity of the system with error range.
2) Regression analysis of the relation between the energy change
by dissolution and an amount of substance.
3) Determine heat of dissolution.
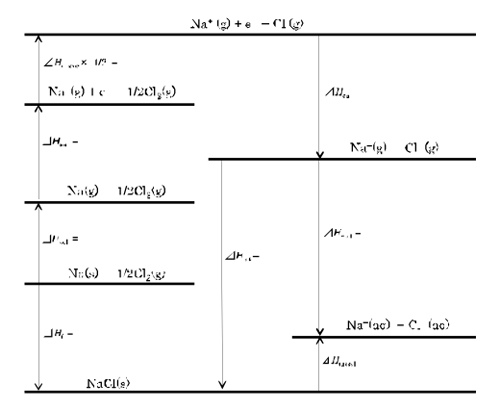
4) Draw the Born-Harbor cycle with the enthalpy change of dissolution.
5) Calculate the enthalpy change of hydration from the Born-Harbor
cycle.
6) Calculate the entropy change of hydration and estimate the
hydration number.
7) Calculate the Gibbs functions of the dissolution and the hydration.
After carrying out the statistical work of the data obtained from the experiment and determination of heat of dissolution, students were expected to obtain relative position of their respective experimental result by creating the Born-Harbor cycle, followed by estimation of the hydration number from the value of entropy change of hydration, and the Gibbs functions. It is constituted so that handling of the thermodynamic parameters can be mastered.
Creation and presentation of the report based on the subject are required in the interview form within the office hours of the instructor, and each experiment completes with acceptance of the report. Degree of comprehension (the principle and thermodynamic theory of the thermal measurement) and acquisition skill (the statistical work of data, a chart, and report writing), and discussion capability (questions and answers about the given subject) are evaluated through this. To the student to whose degree of neither comprehension nor acquisition skill reaches the norm, re-presentation of the report is directed until it reaches the norm.
An example of the obtained result from the experimental program is shown in the following tables. The measuring device (Figure 1) was constituted by the stainless steel Dewar bottle, and form polystyrene lid, the magnetic stirrer, and the electronic thermometer with a data logger (testo-735, resolution: 0.001 °C). Prior to the experiment, the electronic thermometer was calibrated with ice.
Before the measurement of heat capacity of the system, initial temperature of the system, T1, was measured and then 400 cm3 of hot water from the thermostatic bath set to 40 °C was taken and the water temperature was measured, T2. Finally, after putting the hot water into the Dewar bottle, the water temperature was measured, T3. From the average of four data near the median from seven measurements, the heat capacity was determined to be 82.3 JK-1 using the heat capacity of water at T2 as 4.178 JK-1g-1 [3] and density of water at T2 was obtained by interpolation of the data from reference [4] (Table 1). This value was used for measuring the heat of dissolution.

| T1 / K | T2 / K | T3 / K | T3-T1 / K | T2-T3 / K | d / gcm-3 | Cs / JK-1 | ||
| 1 | 289.4 | 314.4 | 313.2 | 23.8 | 1.2 | 0.9913 | 83.7 | 83.7 |
| 2 | 289.4 | 314.7 | 313.7 | 24.3 | 1.0 | 0.9912 | 68.3 | |
| 3 | 289.4 | 314.9 | 313.8 | 24.4 | 1.1 | 0.9911 | 74.8 | |
| 4 | 289.4 | 345.0 | 313.8 | 24.4 | 1.2 | 0.9911 | 81.6 | 81.6 |
| 5 | 289.4 | 315.0 | 313.8 | 24.4 | 1.2 | 0.9911 | 81.6 | 81.6 |
| 6 | 289.4 | 315.3 | 313.9 | 24.5 | 1.4 | 0.9910 | 94.8 | |
| 7 | 289.4 | 314.7 | 313.5 | 24.1 | 1.2 | 0.9912 | 82.6 | 82.6 |
| average | 82.3 |
For measuring heat of dissolution, the sodium chloride and distilled water were stored at the room temperature of about 25 °C for two days prior to use. First, the sodium chloride whose mass was measured was sealed in the sample tube. Then, the tube was put into 400 cm3 of distilled water at T1 in the Dewar bottle. The lid and the electronic thermometer were set immediately after the mixing of the sodium chloride. Measurement was started by agitating the mixture with a magnetic stirrer and T4 was measured. The mass of sodium chloride was changed and five measurements were performed (Table 2).
After confirmation of a linear relationship between the mass of sodium chloride and the temperature change by the dissolution on the graph paper of the laboratory notebook, the data logger was connected to a personal computer to display detailed time course of the temperature change using Microsoft Excel. The extrapolation of the straight line obtained from the detailed time course, initial temperature of dissolution was estimated. The heat of dissolution of the sodium chloride in each amount of samples was plotted against the molality, and by extrapolation, the value at infinite dilution could be read.
| T1 / K | T4 / K | n / mol | Cw / mol kg -1 | T1 - T4 / K | Q / J | _Hsol / KJ mol-1 | |
| 1 | 297.447 | 297.403 | 0.017 | 0.043 | 0.044 | 77.17 | 4.4753 |
| 2 | 297.356 | 297.286 | 0.026 | 0.065 | 0.067 | 117.51 | 4.4996 |
| 3 | 297.438 | 297.350 | 0.034 | 0.086 | 0.088 | 154.34 | 4.4987 |
| 4 | 297.395 | 297.284 | 0.043 | 0.107 | 0.111 | 194.68 | 4.5355 |
| 5 | 297.365 | 297.231 | 0.052 | 0.129 | 0.134 | 235.02 | 4.5471 |
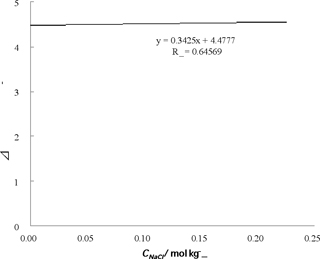
Under this experimental condition, heat of dissolution was determined as 4.48 kJmol-1 (Figure 1). This value was a little larger than 3.88 kJmol-1 in the literature [2]. The Born-Harbor cycle was created, based on the experimental value of heat of dissolution and literature values. The lattice enthalpy was estimated to be -787 kJmol-1 and the hydration enthalpy was calculated as -783 kJmol-1. According to the reference [5], the entropy change of hydration was calculated from the sum of the entropy of monoatomic gas obtained by the Sackur-Tetrode equation [6] and the sum of the entropy in the solution [7]. Finally, assuming the entropy change of hydration as -25 JK-1mol-1 [8], the hydration number was estimated.
The most interesting thing in this experimental program was asked to the students in the interview at the report presentation after the end of this experimental program. As a result, there was an opinion "it was good to actually do the experiment which appears in the textbook of high school chemistry." Although the experimental procedure used in this experimental program is fundamental, it is not conducted commonly at high schools. Moreover, "It was good because with an easy experiment it turned out that a thermodynamic parameter was actually calculated from the experiment", and "The meaning of the thermodynamic parameter was realized". Students' opinion indicated that they were interested in deriving a thermodynamic parameters based on the data obtained from the experiment. In the assignment, the correlation diagram of the thermodynamic parameters in an experimental system was created to visualize relative position of each parameter. The opinion "the reason why the salt dissolve into water came to be found", and the opinion "energy was understood about the relation of the thermodynamic parameter by using a figure, a graph, and a formula" may also indicate that the thermodynamic parameters have been mastered with the concrete image.
An experiment program titled "Determination of heat of dissolution of NaCl" was developed for an undergraduate physical chemistry laboratory class of the third year level students. The program is composed of four parts: i) determination of heat of the dissolution to water; ii) statistical data processing using a spreadsheet-type program on personal computer; iii) calculation of enthalpy change of the hydration; and iv) estimation of the hydration number by entropy calculation. The experimental program provides the students with experiments including the statistical procedure of data. The exercise of thermodynamics was attained by combining the simple experiment, measurement of the heat of dissolution of sodium chloride with the Born-Harbor cycle, and the entropy calculation. Students' response indicated that they were interested in deriving thermodynamic parameters based on the data obtained from the experiment. It may also indicate that the thermodynamic parameter has been mastered with the concrete image.

a The value of SNa+(g) + SCl- (g) was estimated by using the Sackur-Tetrode equation (Nihon Kagakukai ed., "Kagaku Sosetsu 11 Ion to Yobai", Gakkai Shuppan Center, Tokyo (1976), p. 81). b ΔSdissol was estimated using Ksp = 37.79 mol2dm-6 from the solubility 26.43 g(100g)-1 in the reference (Nihon Kagakukai ed., "Kagaku Binran Kisohen 3rd ed. II", Maruzen, Tokyo (1984), p. 173). c Cp = 49.69 Jmol-1K-1 (Nihon Kagakukai ed., "Kagaku Binran Kisohen 3rd ed. II", Maruzen, Tokyo (1984) , p. 243). The number of hydration can be calculated from dividing the value of ΔShyd by -25 JK-1mol-1 of entropy change in hydration (Nihon Kagakukai ed., "Kagaku Sosetsu 11 Ion to Yobai", Gakkai Shuppan Center, Tokyo (1976), p. 83).