E-mail: monroem
Chemical Education Journal (CEJ), Vol. 16/Registration No.
16-101/Received July 17, 2013.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
This paper is a discussion about boiling points of Groups
14 to 17 binary hydrides from the perspective of trends within
a group and within a period. Binary hydrides within a group have
the same molecular structure and an increase in the number of
electrons and boiling points "going down the periodic table",
while those within a period have the same number of electrons,
different structures and an irregular boiling point trend. When
predicting relative boiling points within a group, use molecular
size and number of electrons, whereas, for predictions within
a period, use the concept of assigned number of nearest neighbour
molecules (coordination number) and polarizability.
Key Words: Secondary Education, First Year University,Inorganic
Chemistry, Intermolecular Forces of Attraction, Hydrogen Bonding,
Physical Properties
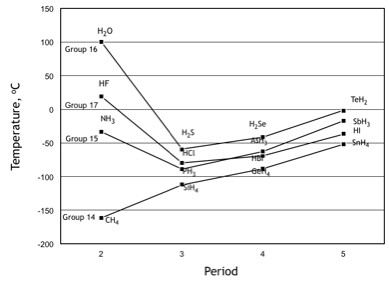
In secondary education and first year university students' chemistry textbooks, there are presentations concerning intermolecular forces of attractions [1-8]. Textbook discussions about these forces of attraction are typically separated into hydrogen bonding and van der Waals forces of attraction and usually have a diagram (see Figure 1) with boiling points [9] of simple binary molecules as examples for ease of teaching. A hydrogen bond is formed when the circumstance arises that a hydrogen atom, which is covalently bonded to either a fluorine, oxygen or nitrogen atom, in one molecule is simultaneously attracted to non-bonding electrons of either a fluorine, oxygen or nitrogen atom that is covalently bonded in another molecule. This type of bonding requires very close neighbouring molecules. Hydrogen bonding has stronger forces of molecular attraction than van der Waals forces of attraction and accounts for unusually high molecular boiling points.
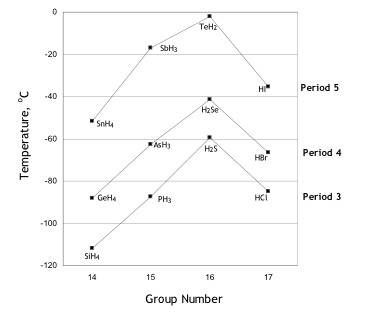
For non-hydrogen bonded binary hydrides boiling points listed in Figure 1, students are usually given the guideline that within a group, boiling point of molecules increases with size and number of electrons. However, a guideline for a boiling point trend of binary hydrides within a period (Figure 2) is not presented in textbooks. This paper focuses on developing such a guideline and in-depth discussions about van der Waals forces are required.
There are three distinct forces collectively known as van der Waals forces which contribute to the long-range interaction among molecules and have been discussed in many textbooks [2, 3,7 and 8]:
London interactions are involved with such phenomena as adhesion, surface tension, physical adsorption, properties of gases and liquids, as well, as adding to forces of orientation. Dispersion forces at times can not only rival but exceed the impact of permanent dipole forces on boiling points; for example, using just bond polarity, we would predict that HCl, with its more polar bond and thus greater molecular polarity (permanent dipole-permanent dipole interaction), would boil at a temperature higher than HI, a molecule with less bond and molecular polarity. However, boiling points are opposite this prediction due to the impact of van der Waals forces of attraction on HI, see Table 1 [10]. In fact the boiling point of HI(-35.5 °C) rivals that of NH3(-33.4 °C), a molecul e with hydrogen bonding and some van der Waals forces of attraction. We may use these examples to demonstrate to students that "weak" van der Waals (vdW) forces can have substantial consequences.
| Molecules | Permanent dipole Moment (Debye) | Polariz- ability (10-24 cm3) |
Cinduction (10-79 J m6) |
Cinduction (10-79 J m6) |
Cdispersion (10-79 J m6) |
Sum CvdW | % Cdispersion to Total CvdW | Boiling Point (°C) |
| Ne-Ne | 0 | 0.39 | 0 | 0 | 4 | 4 | 100 | -246.1 |
| CH4-CH4 | 0 | 2.60 | 0 | 0 | 102 | 102 | 100 | -161.5 |
| HCl-HCl | 1.08 | 2.63 | 6 | 11 | 106 | 123 | 86 | -85.1 |
| HBr-HBr | 0.78 | 3.61 | 4 | 2 | 182 | 189 | 96 | -66.4 |
| HI-HI | 0.38 | 5.44 | 2 | 0.3 | 370 | 372 | 99 | -35.6 |
| NH3-NH3 | 1.47 | 2.26 | 10 | 38 | 63 | 111 | 57 | -33.4 |
| H2O-H2O | 1.85 | 1.48 | 10 | 96 | 33 | 139 | 24 | 100 |
Polarizability arises, in-part, from valence electrons that are non-bonding electron pairs (lone pair electrons) --see Table 2. Within a group listed in Figure 1, as size of atoms increases, effective nuclear charge decreases and effect of polarizability of lone pair electrons on boiling points increases [11].
| Period | Group 14 | Group 15 | Group 16 | Group 17 |
| 2 | C --- |
N 1.0 |
O 0.5 |
F 0.2 |
| 3 | Si --- |
P 2.1 |
S 1.5 |
Cl 0.8 |
| 4 | Ge --- |
As 3.3 |
Se 2.2 |
Br 1.2 |
| 5 | Sn --- |
Sb 3.9 |
Te 2.8 |
I 1.8 |
Using halogen acids listed in Table 1 as a focal point, we can show students a direct correlation between dispersion forces and polarizability, i.e., as one value increases so does the other. This paper now focuses on polarizability as an indicator of dynamic dispersion forces of attraction since polarizability is more accessible in reference books than van der Waals free energy coefficients of dispersion forces,.
Table 3 correlates boiling points within a group to total number of electrons and lone pair electrons, as well as, with polarizability within a group [12]. A simple guideline for students is that as the total number of electrons, in molecules within a group, increases so does the boiling point (Figure 1). Comment: the guideline that as molecular masses increase within a group so do boiling points is unsuitable as it speaks to gravitational fields and is mute on intermolecular forces of attraction; for some students, mass is the explanation for variation in boiling points.
However, Table 3 does not explain
the boiling point trend shown in
Figure 2. In Figure
1, all molecules in the same group have similar molecular
structures and the same number of non-bonding electron pairs while,
in Figure 2, molecules within a period
have dissimilar molecular structures and varying number of non-bonding
electron pairs. See Table 4.
| Molecule | Number of Electrons[12] | Number ofl one pair electrons | Covalent Radius (nm) of central atom [12] | Polarizability (10-24
cm3) [13, 14] |
Tbp (°C) [9] |
| Group 14 | |||||
| SiH4 | 18 | 0 | 0.111 | 4.7(a) | -111.9 |
| GeH4 | 36 | 0 | 0.122 | 5.3(a) | -88.1 |
| SnH4 | 54 | 0 | 0.141 | ----- | -51.8 |
| Group 15 | |||||
| PH3 | 18 | 1 | 0.106 | 4.84 | -87.6 |
| AsH3 | 36 | 1 | 0.12 | 5.4(a) | -62.5 |
| SbH3 | 54 | 1 | 0.14 | ---- | -17 |
| Group 16 | |||||
| H2S | 18 | 2 | 0.102 | 3.86 | -59.6 |
| H2Se | 36 | 2 | 0.116 | 4.7(a) | -41.3 |
| TeH2 | 54 | 2 | 0.136 | ---- | -2 |
| Group 17 | |||||
| HCl | 18 | 3 | 0.099 | 2.63 | -85.1 |
| HBr | 36 | 3 | 0.114 | 3.61 | -66.4 |
| HI | 54 | 3 | 0.133 | 5.44 | -35.6 |
| Period | Group 14 | Group 15 | Group 16 | Group 17 |
| 2 | Tetrahedral (0) | Pyramidal (1) | Bent (2) | Linear (3) |
| 3 | Tetrahedral (0) | Pyramidal (1) | Bent (2) | Linear (3) |
| 4 | Tetrahedral (0) | Pyramidal (1) | Bent (2) | Linear (3) |
| 5 | Tetrahedral (0) | Pyramidal (1) | Bent (2) | Linear (3) |
To help explain the boiling point trend within a period, the issue of nearest neighbours, i.e., coordination number, needs to be discussed. Using hydrogen fluoride, water and ammonium as models, we can remind students that these molecules are assigned, respectively, two, four and two hydrogen bonds.
Hydrogen fluoride: One hydrogen bond derives from a single hydrogen atom and the other from one of the three non-bonding electron pairs (lone pairs) at a given moment; why HF has only two hydrogen bonds and not three or four is, at present, a conundrum [15, 16, 17]. The two hydrogen bonds for hydrogen fluoride mean that there are only two closest neighbouring molecules (coordination number of two) and forms a two dimensional zigzag arrangement of molecules [18]. For the three dimensional network of liquefied HF, two dimensions (e.g., x and y planes) are a function of hydrogen bonding and the third dimension (z plane) by weaker hydrogen bonding or permanent dipole-permanent dipole forces of attraction associated with the more distant molecules [19]; as interstitial spacings among molecules increase, impact of forces of attraction decrease. The remaining binary hydride molecules of Group 17 are linear structures and molecules with the same physical structure have the same coordination number [19]. Thus, HCl, HBr and HI have a coordination number of two and follow the same three dimensional pattern of attraction as liquefied HF pattern of attraction as liquefied HF.
Water is a bent molecule that, in the liquid phase, has a maximum of four hydrogen bonds at any given moment-- two hydrogen bonds from two covalently bonded hydrogen atoms and two hydrogen bonds via two sets of lone pair electrons. Water molecules form a three dimensional array of hydrogen-bonded molecules, has a coordination number of 4, two more than HF, and thus more effectual forces of attraction resulting in a high boiling point. We can assume that H2S, H2Se and H2Te are all bent molecules, have a coordination number of four and follow the three dimensional attraction pattern of liquid water. Now, we can state that a Group 16 molecule in a period has greater collective three dimensional forces of attraction and a higher boiling point than a Group 17 molecule in the same period.
Ammonia has a trigonal pyramidal structure and is limited to two hydrogen bonds [20, 21]. One hydrogen bond is via its single lone pair electrons and the second from any one of the three hydrogen atoms at a given moment. Liquid ammonia molecules form a zigzag line of coordinated molecules and follow the three dimensional pattern of attraction as that of hydrogen fluoride. This discussion of structure, coordination number and three dimensional forces of attraction can be extended to the remaining hydride molecules in Group 15.
A simple guideline for the boiling point trend for binary hydrides within a period is coordination numbers rather than polarizability. See Figure 3. Note, that molecules within the same period (Figure 2) that have the same assigned coordination number have comparable boiling points; the exception is period 5, where data on forces of attraction are questionable [14].
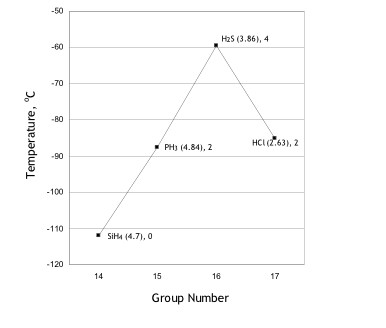
In reference to Group 14, Table 4, the forces of attraction are quite low for non-polar methane, there is a lack of closest neighbours, and it has an extremely low boiling point. This viewpoint can be broadened to the remainder of binary hydrides in Group 14.
This paper was designed to invite students to visualize boiling point trends from the perspective of Group numbers and periods. In order to accomplish this task, students need to comprehend: