2Faculty of Chemistry, Urmia University, 57159, Urmia, Iran
E-mail: nnp403
Chemical Education Journal (CEJ), Vol. 16/Registration No.
16-102/Received December 11, 2013.
URL =
http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract
In the present educational work, a new and simple method to
drawing of the monosaccharide Fischer projection based on new
monosaccharide's barcodes has been presented. In this method,
monosaccharide column, monosaccharide visions, new barcodes for
each monosaccharide (aldose and ketose) is presented and are compared
with known method of Arita & Tokimatsu method. New method
for the boxation of monosaccharide column and corresponding numberations
are exhibited. The Table of monosaccharide is presented based
on new monosaccharide barcodes. Finally, an interesting triangular
diagram for aldoses and ketoses is presented based on new monosaccharide
barcodes.
Keywords: Monosaccharide number, Monosaccharide
vision, Monosaccharide column, Monosaccharide lines, Fischer projection,
Aldose, Ketose
(a) Introduction to monosaccharide column via known Fischer projections.
(b) Introduction to monosaccharide vision via Fischer projections.
(c) Introduction to right and left hands functions in monosaccharide column.
(d) Introduction to boxation of the right and left hands functions and its side.
(e) Introduction to numbering of boxation and its side.
(f) Introduction to monosaccharide's number (monosaccharide's barcode).
Monosaccharide's number recognition
(h) Drawing the Fischer linear projection.
(i) Introduction to triangular diagram for aldoses and ketoses.
Monosaccharides are polyhydroxy aldehydes or ketones having four to nine carbon atoms in their carbon chains most often, five or six [1]. Many chemists have been described new methods to alleviate the knowledge obstacle in monosaccharides. For example: the basic work of Fischer and Van't Hoff in carbohydrate chemistry [2], the use of schematic formulas as an aid to rapidly representing configurations for monosaccharides [3], binary notation to indicate the structure of carbohydrates [4], the history of the nomenclature of carbohydrates [5] have been presented.
Although the structure of carbohydrates is much more complicated
than linear DNAs and protein sequences, linear systematic codes
have been already proposed [6,7]. The monosaccharides, the building
units of carbohydrates, have multiple stereocenters. The presences
of these multiple stereocenters contribute to the rich structural
diversity of carbohydrates, enabling them to serve as 'molecular
(cellular) barcodes' [8]. Monosaccharide
cycles (MoCycle) is an interesting method to determine
the general stereochemical relationships of both D and L monosaccharides
has been reported by Hunsen [8].
Arita and Tokimatsu [9] reported that
the stereo parities of four chiral positions (from C5 to C2) for
D-hexose and D-pentose sugars. They pointed that the corresponding
parity for L-sugars can be obtained by flipping all 1 and 2. In
their method, the parity 1 and 2 corresponds to right and left
orientation of the Fischer projection from the bottom up, respectively.
Non chiral positions are skipped and the keto group is represented
by '_' to distinguish hexoses and pentoses by the same authors.
The mentioned above parity description from the bottom-up is original
notation (Table 1) [9].
In the present work, we have introduced a new and simple method for description of the drawing of the monosaccharide Fischer projection with using new formal barcodes, introducing monosaccharide column, monosaccharide vision, numberation side and triangular diagram for aldoses and ketoses.
|
|
||||
|
(8 patterns) |
allose |
glucose |
gulose |
galactose |
|
(8 patterns) |
altrose |
idose |
mannose |
talose |
| ketohexose (4 patterns) |
111_ psicose |
112_ fructose |
121_ sorbose |
122_ tagatose |
|
(4 patterns) |
ribose |
xylose |
arabinose |
lyxose |
|
(2 patterns) |
ribulose |
xylulose |
This paper describes a new educational method for drawing of
the monosaccharide Fischer linear projections and is useful for
learning of undergraduate and graduate students and introduction
to monosaccharide number and some useful aspects as below;
(a) Introduction to monosaccharide column via known Fischer projections.
(b) Introduction to monosaccharide vision via Fischer projections.
(c) Introduction to right and left hands functions in monosaccharide
column.
(d) Introduction to boxation of the right and left hands functions
and its side.
(e) Introduction to numbering of boxation and its side.
(f) Introduction to monosaccharide's number (monosaccharide's
barcode).
(g) Introduction to monosaccharide Table.
(h) Drawing the Fischer linear projection.
(i) Introduction to triangular diagram for aldoses and ketoses.
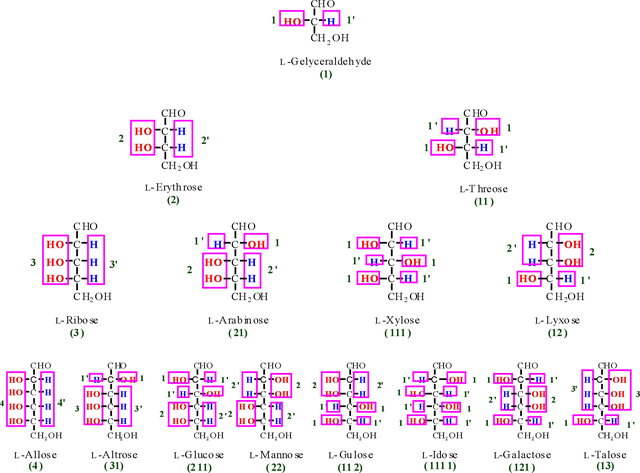
Monosaccharides divided to two classes of an aldose and ketose. Representatively, herein, we discussed about glucose as an aldose and fructose as a ketose. Herein, the perpendicular carbon chain in the Fischer linear form is called monosaccharide column. For instance, the monosaccharide column for D- and L-Fischer projections for four monosaccharides are shown as below in violet perpendicular rectangle.
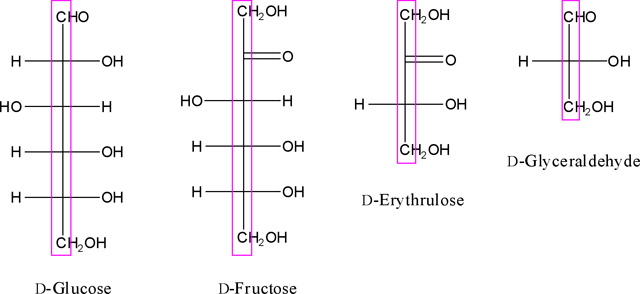
In the monosaccharide molecule, after finding the anomeric functional group in the chain (aldehyde or ketone function), we always look at the monosaccharide column in which the aldehyde or ketone functional group points away from the viewer (Figure. 2).
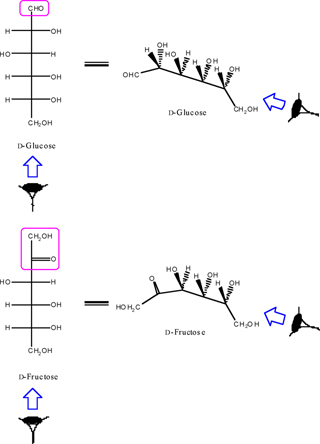
We identify the right and left handed functions in monosaccharide column after we look at the molecule along monosaccharide vision side. According to the Figure. 3, all horizontal functions in the right and left handed of the monosaccharide column (Functions are located on the chiral carbon atoms of aldose and/or ketose) are called column right and left functions, respectively.
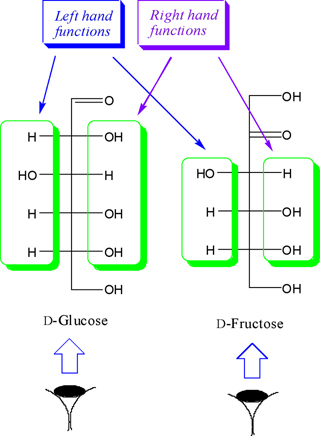
•- The boxation of the monosaccharide column is done for
its column right and left functions.
•- We bundle the right and left hand functions on each chiral
center separately.
•- All H and OH functions to be bundled separately.
•- Any vicinal repeated similar functions are put in one
box (Figure.4).
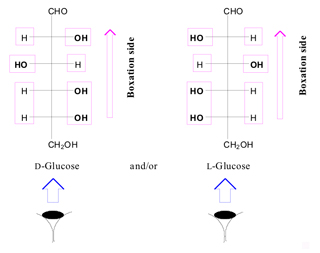
(e) Introduction to numbering of boxation and its side
In Arita and Tokimatsu method [9]
(see also Table 1), they pointed that
the corresponding parity for L-sugars can be obtained by flipping
all 1 and 2. In their method, the parity 1 and 2 corresponds to
right and left orientation of the Fischer projection from the
bottom to up, respectively. In our new method, the barcoding of
each monosaccharide can be done based on the following items.
•- The side of numberation of boxes is the same side of monosaccharide
vision.
•- All boxes contain OH and/or H function to be numbered
by an integer number and primate integer number ('), respectively
and these numbers assigned in green color in the monosaccharide
structure formula (Figure. 5).
•- The number of the functions that are located into each
box is written in the front of each own box (Figure.
5).
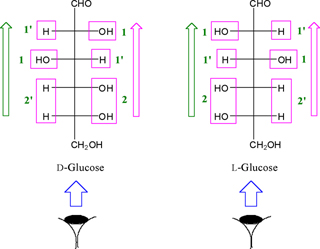
(f) Introduction to monosaccharide's number (monosaccharide's barcode)
From the numberation of monosaccharide left and right handed functions (for example in D- and L-glucose), we can obtain two triplex number (for example; two significant digits for each of D- and L-glucose). For instance, these numbers is shown for D- and L-glucose in Figure. 6 and highlighted as green color into the yellow rectangular.
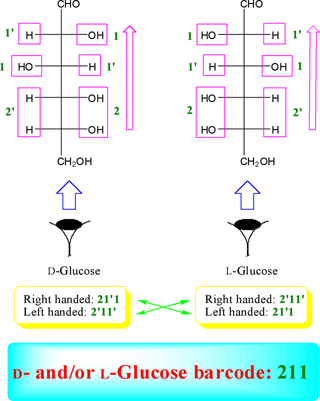
(g) Introduction to monosaccharide Table
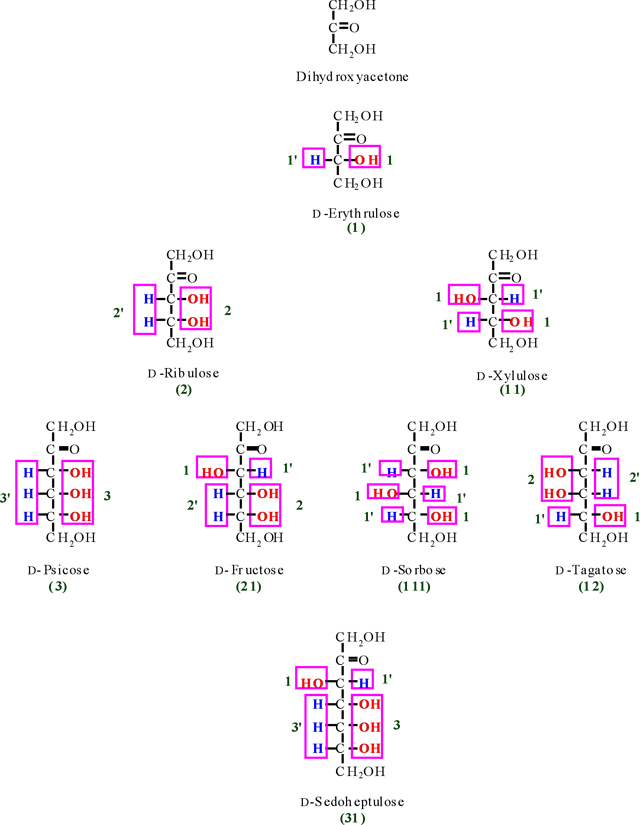
According to Figure. 6, we obtain two numbers of 21'1 and 2'11' for D- and L-glucose that are alternate integer number and primate integer number (the integer and primate integer numbers are corresponding to OH and H functions, respectively). Now, if we waiver from the primate sign, we obtain a significant number of (211) for glucose as representative. Therefore, the number of 211 is called a glucose monosaccharide's number (monosaccharide's barcode). Based on the finding of glucose monosaccharide number, all the related barcode of aldoses and ketoses were obtained and outlined in Table 2 (see also appendixes I-IV).
| Aldose | Aldose code (mnb) |
Ketose | Ketose code (mn) |
| Glyceraldehyde | 1 | Dihydroxyacetone | 0 |
| Erythrose | 2 | Erythrulose | 1 |
| Threose | 11 | Ribulose | 2 |
| Ribose | 3 | Xylulose | 11 |
| Xylose | 111 | Psicose | 3 |
| Lyxose | 12 | Sorbose | 111 |
| Arabinose | 21 | Tagatose | 12 |
| Allose | 4 | Fructose | 21 |
| Idose | 1111 | Seduheptulose | 31 |
| Mannose | 22 | ||
| Gulose | 112 | ||
| Galactose | 121 | ||
| Glucose | 211 | ||
| Talose | 13 | ||
| Altrose | 31 |
Therefore, from the combination of monosaccharide vision side with boxation and numberation sides, the following sign can be obtain in result (Figure. 7).
As we see in Table 2, some of monosaccharides from aldose and ketose groups have the same monosaccharide's no. (For instance; glyceraldehyde and erythrulose (1), erythrose and ribulose (2), Xylose and Sorbose (111) and etc. For the clarification of each aldose's and ketose's no., let us to use a common no. in which to add the letters of A and K for the front of aldose and ketose no., respectively, as bellow;
| Aldose | mnA |
| Ketose | mnK |
Where, mn is the monosaccharide's number, A and K are represents
of aldose and ketose, respectively.
For example: Arabinose no. = 21A Fructose no. = 21K
Threose no. = 11A Xylulose no. = 11K
In the section (f), we discussed the conversion of Fischer projection to monosaccharide number. Now we learn the drawing Fischer projection with using monosaccharide number. On the other words, how we can use the monosaccharide number for drawing the Fischer projection.
The rules of drawing Fischer projection using monosaccharide
number are as follow;
•- Characterize the kind of monosaccharide (aldose or ketose)
according to monosaccharide Table (Table
2).
For example: D-Glucose no.: 211A
D-Fructose no.: 21K
•- Determine the number of carbon atoms in the monosaccharide
chain.
a) In aldoses, the sum of monosaccharide no. boxes plus 2 is equal
to the carbon atoms number in aldose chain skeleton (monosaccharide
no. are available from Table 2), so
as Equation (1);
Where; CA = Number of carbon atoms in
aldose chain, b = box.
Example 1: We can write for Glucose (211A) according to equation
(1); CGlucose = 2 + 1 + 1 + 2 = 6 (indicating
aldohexose)
Example 2: Threose (11A); CThreose = 1
+ 1 + 2 = 4C (indicating aldotetrose)
Example 3: Idose (1111A); CIdose = 1 +
1 +1 + 1 + 2 = 6C
Example 4: Lyxose (12A); CLyxose = 1 +
2 + 2 = 5C
Example 5: Allose (4A); CAllose = 4 + 2
= 6C
b) In ketoses, the sum of monosaccharide no. boxes plus 3 is equal
to the numbers of carbon atoms in ketose chain skeleton as in
Equation (2). Therefore,
Where; CK = Number of carbon atoms in
ketose chain, b = box.
Example 1: Fructose (21K); 2 + 1 + 3 = 6C (indicating ketohexose)
Example 2: Psicose (3K); 3 + 3 = 6C (indicating ketohexose)
Example 3: Erythrulose (1K); 1 + 3 = 4C (indicating ketotetrose)
Example 4: Dihydroxyacetone (0K); 0 + 3 = 3C
c) The route of drawing Fischer projection using simple monosaccharide
number.
For instance, for D-glucose, in the opposite side of monosaccharide
vision, placing a hydroxyl group (OH function) at C-2 to the right
(for number 1) and next OH function to the left (for number 1),
then two vicinal OH (for number 2) to the right in the alternate
horizontal bonds in monosaccharide chain (Figure.
8).
In the other hands, the barcode of 211A (D and/or L-glucose), the number 1 (in blue color) is the hydroxyl group in which near to the aldehyde functional group on the first chiral carbon atom in the monosaccharide column chain. The number of 2 (in red color), indicating the two vicinal OH groups to be far (from aldehyde functional group) in the left and/or right-hand of monosaccharide column (Figure. 8). Based on this rule we can draw all monosaccharide (aldose and ketose) Fischer projection from their corresponding barcode. For example: For the drawing of the Fischer projection of D- and/or L-Glucose (211A) and D- and/or L-Fructose (21K), it can be proceeding as below:
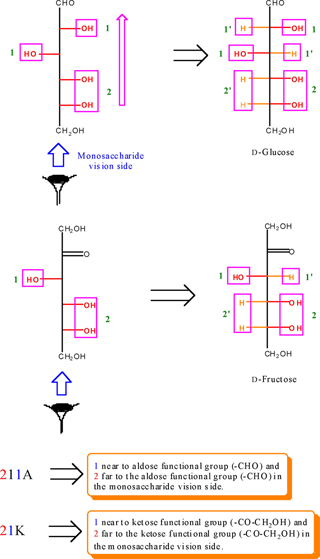
d) Draw the chain after determining of the number of carbon
atoms in the monosaccharide chain.
d-1) In aldoses, draw CHO group as a C-1 and refer the CH2OH as a latest carbon atom (Figure.
9).
d-2) In ketoses, refer the -CO-CH2OH group
as a C-1 and C-2, respectively, and CH2OH
as a latest carbon atom (Figure. 9).
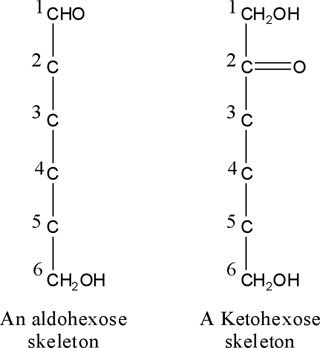
d-3) Look at the molecule in monosaccharide vision side.
d-4) Locate the hydroxyl and/or H functions in the chain with
using monosaccharide number.
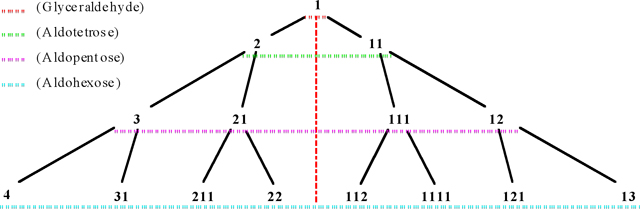
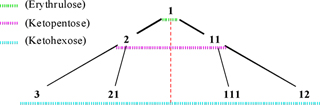
Interesting triangular diagrams of aldoses and ketoses are shown in Figure. 10 and Figure. 11, respectively. According to these diagrams and appendixes I-IV, the learning and remember of monosaccharide's number to be easy in this method.
In summary, a new and simple method to drawing of the monosaccharide Fischer projection based on new monosaccharide's barcode has been presented. The new monosaccharide's barcode caused to become easy learning and mnemonic of the monosaccharide's Fischer projection. The drawing of any monosaccharide stereo structure (aldose and/or ketose) to be very easy according to new barcodes and is the advantage of this new method.
We gratefully acknowledge the Research Council of Urmia University.
Appendix I
Tree diagram of D-ketoses and their codes (Codes of D- and
L- stereoisomers are the same).
Appendix II
Tree diagram of L-ketoses and their codes.
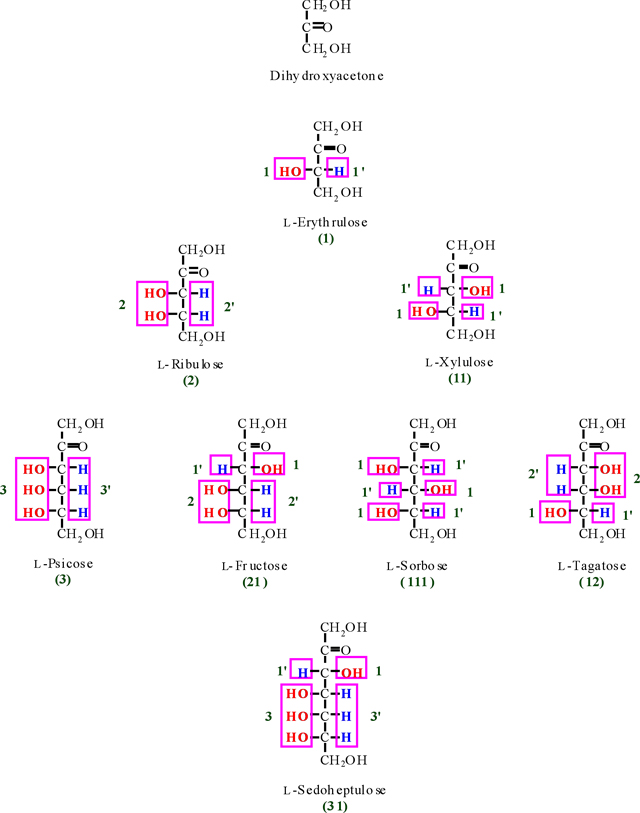
Appendix III
Tree diagram of D-aldoses and their codes (Codes of D- and
L- stereoisomers are the same).
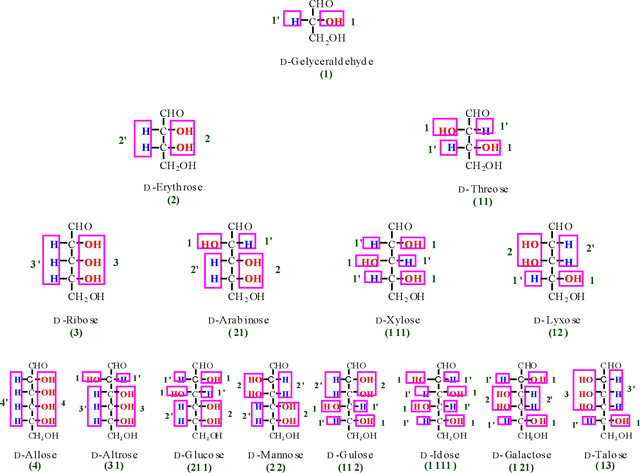
Appendix IV
Tree diagram of L-aldoses and their codes.