Chemical Education Journal (CEJ), Vol. 17/Registration No.
17-102/Received March 5, 2015.
URL = http://chem.sci.utsunomiya-u.ac.jp/cejrnlE.html
Abstract (Resumen)
The construction and usage of a paper helicopter in the teaching-learning
of P/M-chiral descriptors are presented. These descriptors
are used to characterize helical molecules such as helicenes and
liquid crystals.
(Se presentan la construccion y el uso de un helicoptero de papel
en la enseñanza-aprendizaje de los descriptores quirales
P/M (Plus/Minus). Estos descriptores se usan en la caracterizacion
de moleculas helicoidales tales como los helicenos y los cristales
liquidos.)
Keywords: Paper helicopter, helical chirality, P/M-chiral descriptors, hands-on activity, teaching stereochemistry
The construction of a paper helicopter
The physics of a paper helicopter
Chirality is the physical property of an object to be non-superimposable with its mirror image. This word is related with the ancient Greek kheir, which means hand. It was introduced in 1904 by Lord Kelvin in his Baltimore lectures on "Molecular Dynamics and the Wave Theory of Light", as follows [1, 2]:
"I call any geometrical figure, or group of points, chiral, and say it has chirality, if its image in a plane mirror, ideally realized, cannot be brought to coincide with itself"
In this context is very important, of course, to mention the work of Louis Pasteur, the father of modern microbiology and a pioneer of stereochemistry. He was capable manually to separate, left and right handed crystals of sodium ammonium tartrate (Figure 1), the so called "racemic mixture" (1:1 mixture of dextro- and levo-rotatory samples of ammonium sodium tartrate) [1, 3].
 |
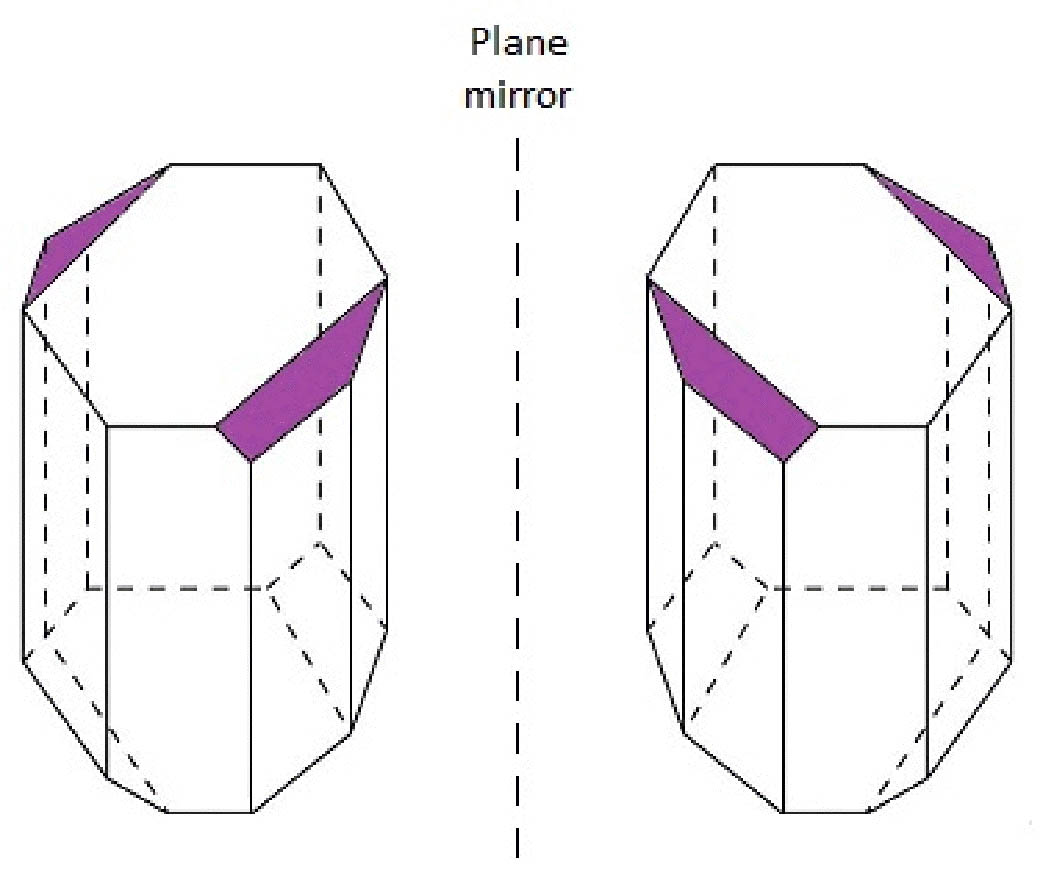 |
Figure 1 Louis Pasteur and a schematic representation of enantiomorphic crystals of (+)- and (-)-ammonium sodium tartrate, which were manually separated by himself [4].
Actually, among chemists, physicians and people related with the synthesis and production of drugs, the term chiral is very popular, and necessary, because more than one-half of marketed drugs are chiral [5]. This fact acquired great relevance since 1962, when ca. 12,000 babies suffered phocomelia due to the consumption of racemic R/S-Thalidomide by their mothers being pregnant ("Thalidomide tragedy"). Although initially was believed and demonstrated that R-enantiomer was sedative for pregnant mothers and S-enantiomer was teratogenic for their babies [6-9]; actually is known that Thalidomide racemices in-vitro as well as in-vivo [10].
There are three types of chirality elements: Chirality center, chirality plane and chirality axis. The existence of at least one of these elements in a molecule makes it chiral, meaning non-superimposable with its plane-mirror image.
Many types of molecules present a chirality axis [11]; however, there are different kinds of descriptors to name each one; for example, chiral descriptors P/M, Ra/Sa and Δ/Λ are mentioned in IUPAC's Gold Book [12]. In particular, in the case of molecular (e.g. helicenes) and supramolecular (e.g. biological) helices is more common the use of P/M-chiral descriptors, whereas in helical coordination compounds (e.g. helicates) Δ/Λ-chiral descriptors predominate.
M (or minus) and P (or plus) chiral descriptors are used respectively, to describe left- and right-handed helices (Figure 2). Since a geometrical point of view, if we take a small part of the left-handed helix then we will observe it possesses a negative slope (m<0); by contrast, a right-handed helix can be described with a positive slope (m>0).
At this point, students must remember that line's slope is defined as:
m = Δy/Δx = (y2-y1)/x2-x1 = rise/run or tan(α),
where α is the corresponding angle in standard position (An angle is in standard position if its vertex is located at the origin and one ray is on the positive x-axis. The ray on the x-axis is called the initial side and the other ray is called the terminal side. The angle is measured in a counter-clockwise direction (Figure 2, bottom) [13]).
Figure 2 Left- and right-handed helices are described with M- and P-chiral descriptors and possess negative (m<0) and positive slopes (m>0), respectively. Mr. Slope and the definition of an angle in standard position are at bottom.
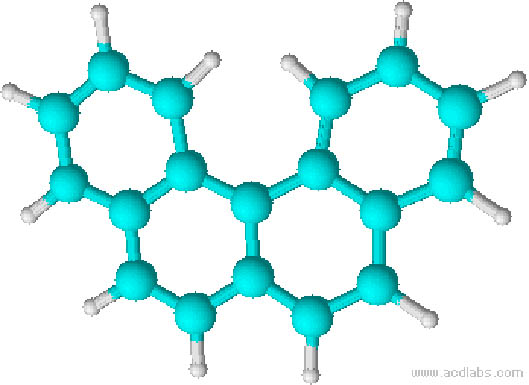
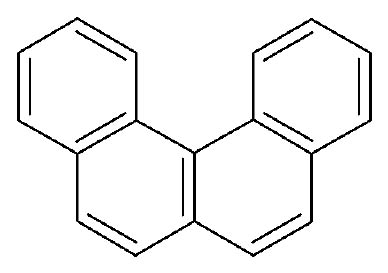
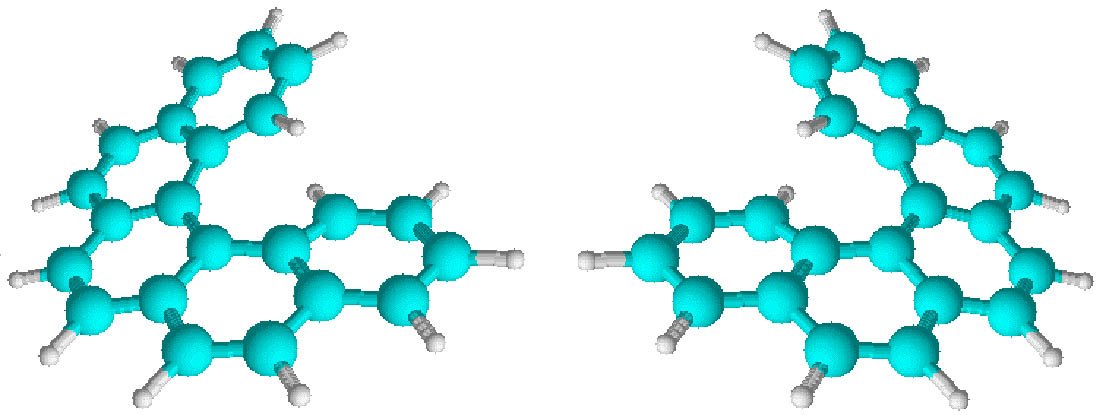
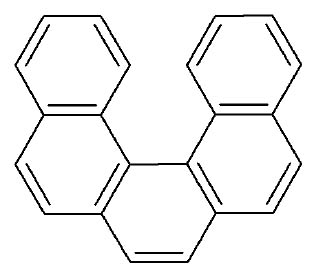
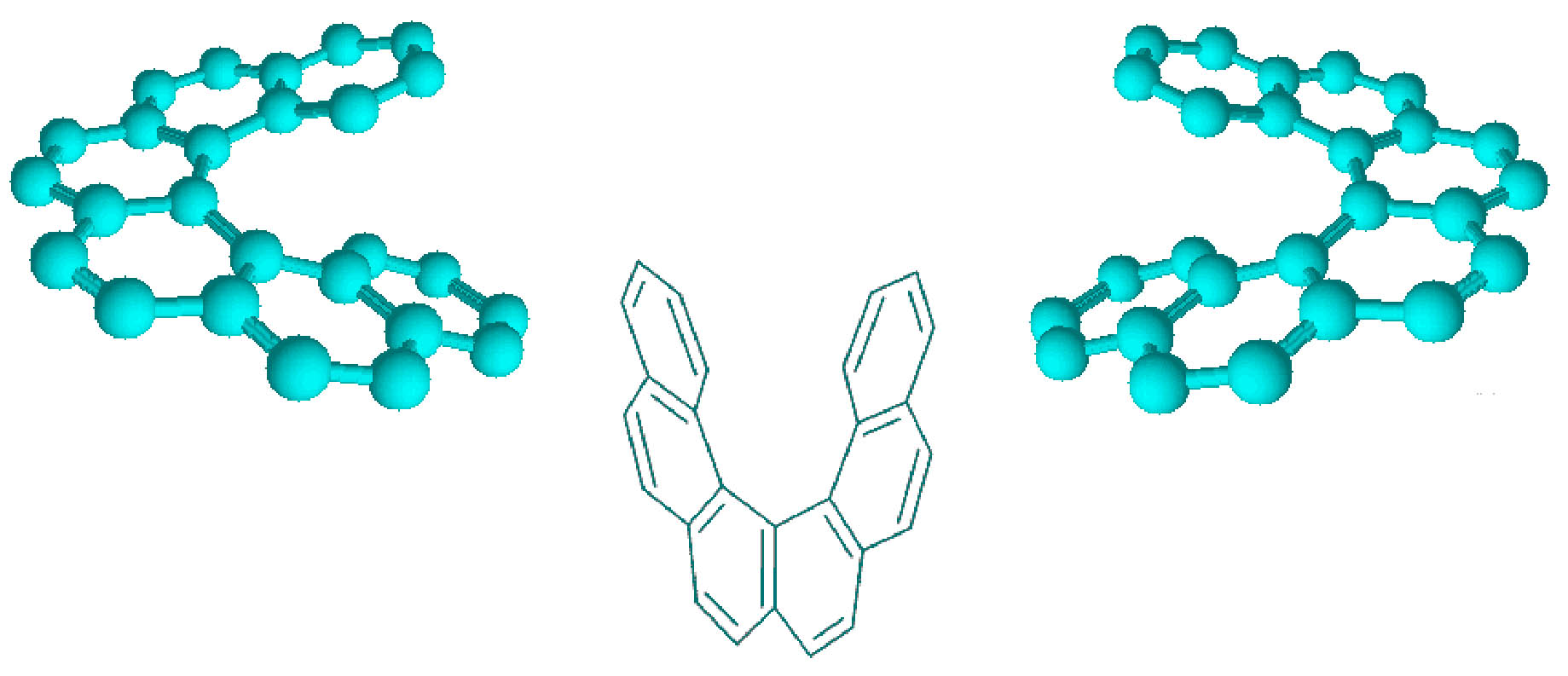
On the other hand, classical examples of molecular helices are helicenes, which are aromatic ortho condensed rings. When the number of rings is higher than four, the system cannot already be planar and adopts a helical structure to liberate the steric congestion. In the case of [5]-helicene the racemization barrier at 27 °C is 24.1 kJ/mol and it increases to 36.2 kJ/mol for [6]-helicene (Figure 3) [14].
  |
  |
 |
|
Figure 3 Line formulas and ball and sticks representations of ortho condensed rings. When the number of ortho condensed rings are higher than four, M- and P-helices are produced (i.e. [5]- and [6]-helicenes).
Although there are several forces acting on a paper helicopter
during its flight, they can be reduced to (Figure
4): a) A downward force due to earth gravitation (g); b) A
couple of forces, perpendicular to its body, that point in opposite
directions; c) An upward force due to minor pressure on the top
of blades (Bernoullie's Theorem [15]).
Once the helicopter's descending begins due to gravity, airflow
is produced between its blades. Because blades are not in straight
line, forces do not cancel but produce a whirlpool effect, whose
clockwise or counter clockwise direction depends on their initial
orientation (Figure 4c). Such orientation
does not change during the flight and blades rise up to an equilibrium
position.
For a better observation of spin direction, we designed three helicopters: A "standard helicopter" (Figure 5); a helicopter possessing broad blades (Figure 6) to reduce its spin velocity and a helicopter showing inclined blades since the beginning (Figure 7). In all cases, enantiomorphic helicopters are provided.
Figure 4 a) Perpendicular view to helicopter's body illustrating the main forces acting on a paper helicopter; b) Top view showing the direction of airflow between blades; c) The whirlpool direction produced by opposed and unaligned forces of airflow.
a) Cut out the templates of Figures
5-7.
b) Crumple the circles in small balls as compact as possible.
c) Observe at this moment that both structures do not fly but
drop in the same manner.
d) Cut along central line a-b and bend the wings in opposite directions
following the instructions into templates labeled as M
and P.
e) Drop (or throw catching the ball of) M-helicopter and
observe the direction it flies. Repeat the same procedure for
P-helicopter. Check that once the planarity breaks (after
bending their blades up to the indicated positions) they become
chiral and enantiomorphic objects from one other (Figure
8).
f) If you look at M-helicopter parallel to paper´s
plane, you will observe its wings have negative slope. This helicopter
flights in counter-clockwise direction describing an M-helix.
By contrast, P-helicopter's wings have positive slopes
and flights in clockwise direction describing a P-helix.
This fact can be correlated with pictures of Figure
2.
g) Terminated models are presented in Figure
8. See video for paper helicopters' flights in slow motion
(download as mp4, time 1:56 to 2:40 minutes) or use models possessing
wide blades (Figure 6) for verifying
by yourself, the directions they flight.
Figure 5 Templates for building standard paper helicopters capable to flight in counter-clockwise (M-helicopter) and clockwise (P-helicopter) directions.
Figure 6 Templates for building paper helicopters possessing wide blades. This fact reduces their velocity and helps to visualize the direction of spin.
Figure 7 Templates for building paper helicopters with inclined blades. This feature helps to stablish the slope's sign of the whirlpool produced by M- and P-helicopters while dropping.
Figure 8 Terminated models of left (M = minus) and right (P = plus) standard helicopters. Note they are enantiomorphic (mirror image each other).
In our stereochemistry and didactic of chemistry courses, we
take apart the spatial configuration of helicenes and related
them with the spatial configuration of paper helicopters, as a
funny hands-on activity for the teaching-learning of P/M-chiral
descriptors (Figure 9a-b).
Although paper helicopter is known since the beginnings of 90's
[16] and it has been used in the teaching-learning
of statistics (Figure 9c) [17,
18], nature has anticipated to man
with their own helicopter seeds, which disperse kilometers far
from tree to ensure its reproduction (e.g. Gyrocarpus
americanus (Figure 9d), an endemic
tree of Oaxaca, Mexico) [19].
Acknowledgement
Special thanks are expressed to Dirección General de
Planeación Institucional, BUAP, for the financial support
to our research group BUAP-CA-263 (Investigación Experimental
y Teórica de Nuevos Materiales y Educación en Ciencias).
 |
 |
 |
 |
[1] Guijarro, A.; Yus M. "The
origin of chirality in the molecules of life: A revision from
awareness to the current theories and perspectives of this unsolved
problem". Royal Society of Chemistry, Cambridge, UK (2008).
[2] Le Guennec, P. "Describing
Chirality" (1999). Available at: <http://www.chiral.com/description/research.htm>.
[3] Mezey, P. G. "New developments
in molecular chirality". Kluwer Academic Publishers, Dordrecht
(1991).
[4] Manchester, K. "Exploding
the Pasteurian legend". Endeavour, 25 (4),
148-252 (2001).
[5] Millership, J.; Fitzpatrick, A.
"Commonly used chiral drugs: a survey". Chirality,
5, 573-576 (1993).
[6] Barber, J.; Rostron, C. "Pharmaceutical
chemistry". Oxford University Press, Oxford (2013), p. 66.
[7] Campbell, B. C. "Disasters,
accidents, and crises in American history: A reference guide to
the nation's most catastrophic events". Facts On File, New
York (2008), p. 309.
[8] Shorthose, S. "Guide to EU
pharmaceutical regulatory law". Kluwer Law International,
Alphen aan den Rijn (2011), p. 4.
[9] Wolf, C. "Dynamic stereochemistry
of chiral compounds: Principles and applications". RSC Pub.,
Cambridge, UK (2008).
[10] Eriksson, T.; Björkman, S.;
Roth, B.; Fyge, A.; Höglund, P. "Stereospecific Determination,
Chiral Inversion In Vitro and Pharmacokinetics in Humans of the
Enantiomers of Thalidomide". Chirality, 7 (1),
44-52 (1995).
[11] Nasipuri, D. "Stereochemistry
of organic compounds: Principles and applications". Wiley
Eastern, New Delhi (1994), chap. 5.
[12] "IUPAC Compendium of Chemical
Terminology", 2nd ed. (IUPAC Gold Book). Compiled by A. D.
McNaught and A. Wilkinson. Blackwell Scientific Publications,
Oxford (1997). Available at: <http://goldbook.iupac.org/A00547.html>.
For Δ/Λ-descriptors see: <http://goldbook.iupac.org/D01511.html>.
[13] Roberts, Donna. "Standard
Position and Reference Angles". Retrieved from: <http://www.regentsprep.org/regents/math/algtrig/ATT3/referenceAngles.htm>
[14] Rajca, A.; Miyazaka, M. in: "Functional
Organic Materials: Syntheses, Strategies and Applications"
by Müller T. J. J., Bunz Uwe H. F., John Wiley & Sons,
Germany (2007), p. 567.
[15] Dingle, L.; Tooley, M. H. "Aircraft
Engineering Principles". Routledge, New York (2013), p. 481.
[16] Box, G. E. P. "Teaching Engineers
Experimental Design with a Paper Helicopter". Report No.
76, Center for Quality and Productivity Improvement, University
of Wisconsin (1991).
[17] Erhardt, E. (2006). Retrieved
from: <http://statacumen.com/pub/Erhardt_slides_RSM_paper_helicopter_20061104.pdf>.
[18] Zholud, D. "The paper helicopter
experiment" (2012). Retrieved from: <http://www.paperhelicopterexperiment.com/design-and-assembly.php>.
[19] Rivera-Hernández, J. E.
"Notas sobre Hernandiaceae: Primer registro de Gyrocarpus
americanus JACQ para Mexico y de Sparattanthelium Amazonum Mart
para Oaxaca". Acta Botanica, enero, numero 078, 67-76
(2007). Retrieved from: <http://www.redalyc.org/articulo.oa?id=57407804>.