Chemical Education Journal (CEJ), Vol. 4, No. 1 /Registration
No. 4-12/Received January 31, 2000.
URL = http://www.juen.ac.jp/scien/cssj/cejrnlE.html
E-mail: t610002@cc.ncu.edu.tw
Abstract: Solid state chemistry has been one of the most extensively studied subjects in the recent two decades. However, in the past, chemistry-major students did not have much training on solid state reactions and the techniques of physicochemical studies on solid state materials. This designed experiment employed a simple red-ox reaction of tungsten oxide (WO3) to demonstrate a typical solid state intercalation reaction and techniques of conductivity measurements. Some general concepts in solid state chemistry, such as intercalation, categorizing of materials based on conductivity, and charge transport properties of solid state materials are also introduced.
At the end of the twentieth century, the rapid progress of science and technology has forced basic science educators to face a great challenge. Namely: how to educate youth both in basic knowledge as well as frontier science and technology in a limited time. The department of chemistry at National Central University was founded recently (1993). Our education and research goals were focused specially on environmental science and materials chemistry. These two important fields of chemistry were also integrated into the curriculum of our undergraduate courses. For example, chemical waste treatment [1]; the preparation of pH sensors; and the intercalation reactions in solid state materials [2] were included in the general chemistry laboratory.
Solid state chemistry was one of the bases of the materials science. In the past, most chemistry departments did not put the solid state chemistry-related experiment, especially synthesis, in the curriculum of undergraduate laboratory courses. This may be because the theory of the solid state materials is difficult for student majores in chemistry and most physicochemical experiments on solid state materials require extra instruments. Furthermore, in the past, traditional solid state chemistry had been regarded as a not quite knowledgeable science , due to the fact that preparation procedures of solid state materials are simple but the products are complicated and unpredictable in nature. Nevertheless, nowadays, solid state chemistry has become one of most extensively studied sciences amongst the chemists.Highly developed science and technology require new materials and new techniques. No matter the preparation of new materials or using new techniques to fabricate old materials, all need the knowledge and skill of solid state chemistry and experiments. In this respect, the training of experimental skills of solid state chemistry is especially important. The intercalation of hydrogen in tungsten oxide, taken from "Teaching General Chemistry: the Materials Science Companion" edited by A. B. Ellis et al. [3], was a good example to demonstrate the typical reaction of solid state materials and the modification of physicochemical and charge transport properties of solid state materials by simple chemical reactions. The preparation procedure is simple and the tremendous changes in the conductivity and color can attract students easily.
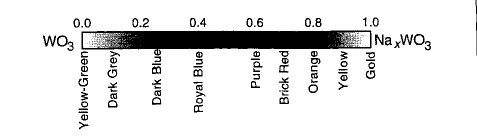
Tungsten oxide (WO3) is a well-known electrochromic (color change by applying electric field) material with diverse colors. The structure of WO3 is built by the corner sharing of WO6 octahedron units. When an atom, such as hydrogen or a metal ion, is inserted in the WO3 structure, the material (MxWO3, M= H, Ca, Sr, Ba ..) is called tungsten bronze. Tungsten bronze owes its name to the metallic luster and is used in the production of bronze paints. It also provides a good example of the formation of intense and characteristic color by the reduction of metal oxides. The corresponding color of MxWO3 in different degrees of reduction of W+6 is shown in Figure 1 [3].

Figure 1: The color of Na xWO3 with different x values (degree of reduction of W).
Furthermore, the charge transport properties of WO3 also change from insulating to semiconducting to metallic conducting upon reducing W+6 and then intercalating foreign atoms. The conducting materials can be roughly divided into semiconductor, metallic conductor and superconductor, based on the temperature-dependent conductivity. The higher the temperature, the higher the conductivity is semiconductor. The conductivity of the metal decreased when the temperature increased. When the materials have no resistance at a certain temperature (generally very low), we call them superconductors. The conducting mechanisms of those conductors may be very complicated. However, a simple chemical reaction (such as reduction-oxidation) can change the material from one type of conductor to another.
The reduction-oxidation between WO3 and Zn
WO3 + x/2ZnWO3x-
+ x/2Zn+2
The intercalation of H+ in WO3
WO3x- + x H3O+HxWO3 + x H2O
The reduction-oxidation between HxWO3 and O2
4HxWO3 + xO24WO3
+ 2xH2O
The conducting property of a material comes from the movement of ions, electrons, or holes inside the material. Therefore, the conductivity of the material depends on the mobility and number of charge carriers. In the reduction of WO3, the W+6 was reduced to W+5, the extra electrons were located in the conduction band of WO3, and therefore increased its conductivity. The resistance (unit: Ohm) and conductivity (unit: Simen/cm or 1/ohm.cm) were commonly used to express the degree of conducting. However, the resistance depends on the size and shape of the materials, therefore, the most commonly used way to express the movement and number of charge carriers in a material is the conductivity.
In this experiment, the set-up for measuring the resistance is shown in Figure 2. The resistance was read directly from the multimeter.
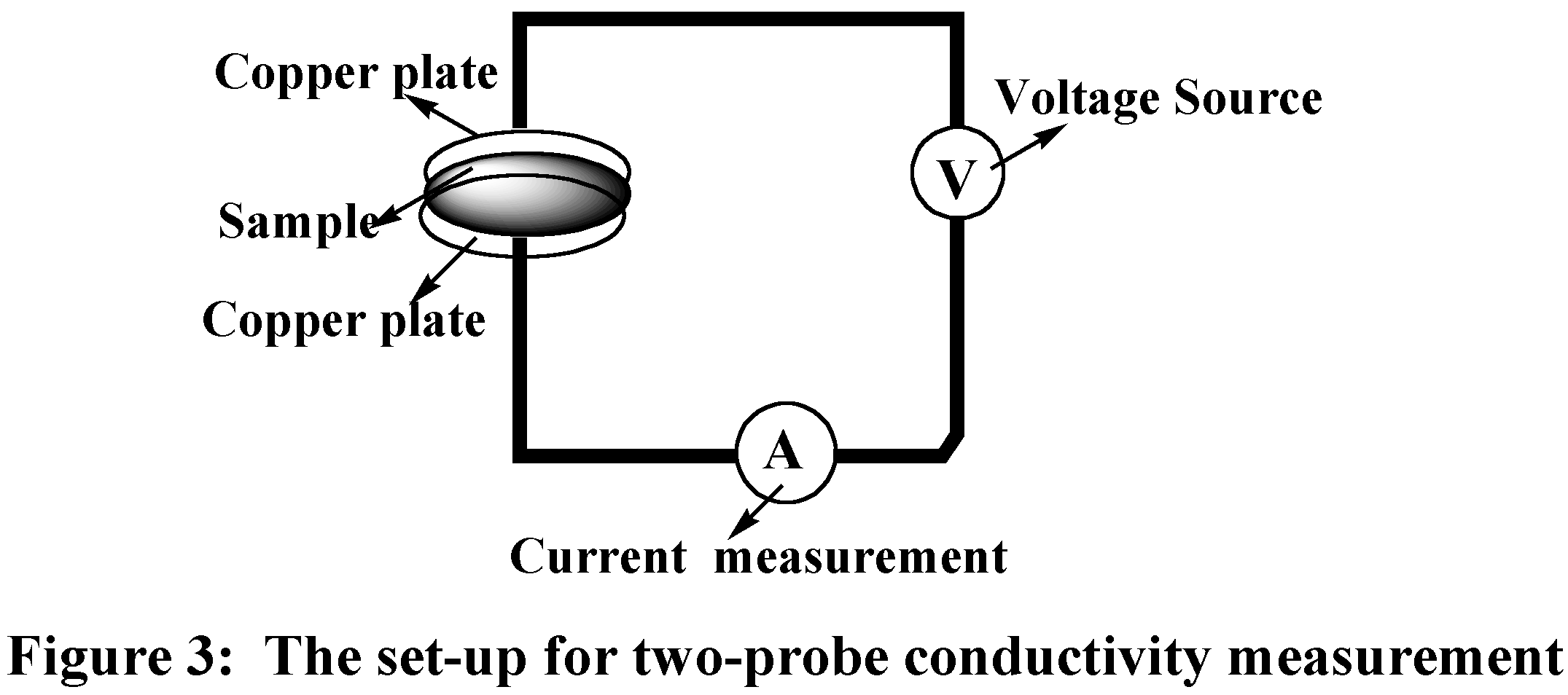
There are many ways to measure the conductivity. The two-probe
method was one of the simplest. The set-up of the two-probe conductivity
measurement is shown in Figure 3
and the conductivity was calculated as follows:
Furthermore, the structure of WO3 changed when the W+6 was reduced to W+5, therefore, the electronic structure also changed. The variation of the energy gap between the HOMO and LUMO of WO3 also changed the color of the material.
(A) Synthesis
(B) Conductivity measurement
(Caution: The packing of the sample and the contact between copper wire and sample will affect the results of the measurement.)
(C) The stability of tungsten bronze
Most of the chemistry freshmen not know the difference between solid state materials and molecular solids; resistance and resistivity; and the intercalation reaction and addition reaction. In addition almost all of the students do not have any experience with solid state reaction. Therefore, a learning evaluation was carried out amongst forty freshmen major in chemistry right after the experiment was done. The results were as fallows:
From this designed experiment, students can learn several concepts and techniques in solid state chemistry, such as the preparation of solid state materials, solid state reactions, reduction-oxidation reactions, conductivity measurement, and the units and physical meaning of conductivity.
 Top
Top

 Header
Header
