Chemical Education Journal (CEJ), Vol. 4, No. 1 /Registration
No. 4-13/Received February 26, 2000.
URL = http://www.juen.ac.jp/scien/cssj/cejrnlE.html
E-mail: chetsf@scc.ntnu.edu.tw
Abstract: The purpose of this study was to unravel problem-solving
strategies, problem-solving activities and the difficulties for
novice science major students in the process of solving the stereochemistry
of coordination compound problems. The study was divided into
two parts, the first part aimed to explore the correlation between
spatial perception ability and stereochemistry problem-solving
ability; the second part was to analyze the science majors' inner
process through the protocol analysis collected from thinking-aloud
and interview methods.
The 45 subjects in this study took paper and pencil tests on ability
of spatial perception and solving stereochemistry problems, and
seven of the students (two high-school, three freshmen science
majors and two sophomores) solved stereochemistry problems with
the thinking-aloud method and were videotaped. After the analysis
of the problem-solving process, the researcher discussed the differences
in behavior, activities, and process in solving stereochemistry
problems between high proficiency and low proficiency students.
The ten problems which were validated by expert professors were
concentrated on geometric isomers, optical isomers, reflection
operator, reversion operator and rotation operator.
The findings suggested that the correlation between spatial perception
ability and stereochemistry problem-solving ability is quite good
with r=.647. On average, the best performance of students was
in rotation operator. It was due to their past learning experience.
The more successful students had the better reasoning strategy.
They were more sensitive to the stereo structures, and realized
the change of the structure more correctly. The less successful
students used worse reasoning strategy and less effective trial-and-error
strategy. The latter had wrong recognition of stereo structures,
regarding optical isomer as geometric isomer; they also had bad
visualizing spatial relationship among atoms.
Cognitive science has been used in the development of spatial problems over the past thirty years. Early works of Newell and Simon [1] provided the impetus for much more research on science problem-solving. The work is based on information-processing theory, and thinking-aloud and interview as the main data-collection technique. Gardner [2] described "spatial intelligence" as one of the seven intelligences. Spatial intelligence is concerned with sensitivities about the spatial correlation, line, shape, style and color. Many studies have indicated that students tend to experience difficulty with many aspects of three-dimensional structures. One of the reasons they failed in learning stereochemistry was due to a lack of "spatial perception ability".
The "Stereo-structure" of molecules is one of the most important concepts in Chemistry and Biology. The ability of students to perform certain spatial operations is, therefore, essential for many courses to explain the physic of and chemical characters. Several studies, however, have indicated that students tend to experience difficulty with many aspects of three-dimensional thinking (Atkinson [3], 1993; Liao [4], 1999; Seddon, Eniaiyeju & Juson [5], 1984).
The purposes of this study were to
As to the study of problem-solving in stereochemistry, only organic compounds have been studied recently by Chiu s group (Liao [4], 1999). The deep and wide angle of inorganic chemistry, especially in ligand coordinated transition metallic compounds were focussed in this study. Since the problems of stereochemistry did not involve calculating, reasoning of spatial relationships and pictorial tasks were focussed for organizing the test problems. Researcher-collated high school chemistry textbook (III) and coordination compounds in undergraduate General Chemistry were used to design stereochemistry test problems. Symmetry with five elemental operators which lead to the development of group theory, is the center of understanding the stereochemistry of the molecule. Three operators, reflection, inversion, and rotation, were included in the design of problems for testing ability in solving stereochemistry problems.
The stereochemistry of a coordination compound is the study of the space arrangement of a compound in which a central metal ion (or atom) is attached to a group of surrounding molecules or ions by coordinate covalent bonds. One of the more interesting aspects of coordination chemistry is the existence of isomers, compounds that have the same formula but a different arrangement of their constituent atoms. Constitutional isomers and stereoisomers are two different categories of isomers. Stereoisomers were focused on in this study.

Conformational isomers were also excluded in this study. Concepts of geometric isomers, optical isomers and operators of reflection, inversion and rotation were employed to organize the test (enclosed in Appendix), which consists of six types of problems, 10 questions. The tool was through the testing of 42 senior high school contestants in the domestic chemistry Olympiad, 43 physics-major freshmen, and 2 chemistry-major graduate students, and then validation through comments of 2 senior inorganic chemistry professors.
The study was divided into two parts. The first part aimed to explore the correlation between spatial perception ability and stereochemistry problem solving ability. Of the 45 who participated in the study, 38 were freshmen majores in Physics. The second part was to analyze the science majors' inner process through protocol analysis collected from thinking aloud and the interview method. Seven of the participants (2 high school students, 3 freshmen majors, 2 sophomore majors, all of whom were males.) were invited by the researcher to take tests using the thinking aloud and interview method. High school students were applying for admission to science college. Freshmen had completed a semester of General Chemistry, and the sophomores had completed two semesters of General Chemistry and a semester of Organic Chemistry. All participants performed thinking-aloud problem solving for each of the 10 questions.
The research of the problem-solving in stereochemistry was documented using the thinking-aloud method. The following procedures were used to ensure reliability, validity, and richness of data collected.
Prior to solving the question, the researcher explained to the students the concepts of reflection and inversion operations which they had never learned before. Then each participant attempted a different and simple warm-up question. All subjects were videotaped as they thought aloud while solving each of ten problems in stereochemistry. The videotape records were reviewed, and protocols were coded following the guidelines of Ericsson and Simons [6]. (1)Encoding vocabulary. (2) Coding the segments.
The 12 common problem-solving strategies( Dhillon [7], 1998; Liao [4], 1999) which were used by the seven students are : Forward strategy, Considering simple case, Trial and error, Random Process, Analogy, Mean-ends analysis, Guessing, Problem abstraction, Problem decomposition, Working backward, Envisioning, Visualizing.
All data collected from either the spatial ability test or the stereochemistry test were treated to get the average, standard deviation and the correlation.
The average score of all subjects in the spatial ability test is 70.23% and standard error is 6.04; The average score in the stereochemistry test is 53.06% and standard error is 20.68 as shown in Table 1. According to the Pearson test, the spatial ability and the stereochemistry achievement have positive correlation.(correlation=.647 , p <.01).
|
Tests |
Spatial Ability Test |
Stereochemistry Test |
|
Average |
22.48 |
53.06 |
|
Spatial ability |
6.04 |
20.68 |
|
Ratio(%) |
70.23 |
53.06 |
The scores for problem-solving were based on the number of correct answers and the reasons provided by the subject for each problem. According to the scores they got the subjects were categorized as successful unsuccessful, the middle one (a high school student) being excluded.
The average score of six thinking-aloud problem solvers is 74.96% as shown in Table 2. The best score among the six types is Type 6 (93.33%), whereas the worst is Type 5 (33.33%) as shown in Table 3, which is the stereochemistry of inversion of a molecule.
|
Problems |
Students |
Hf-1 |
Hf-2 |
Hh-3 |
Ls-1 |
Ls-2 |
Lf-3 |
Average |
|
Type l |
(1-1) |
10 |
10 |
10 |
10 |
10 |
8 |
9.67 |
|
(1-2) |
10 |
10 |
10 |
6 |
6 |
6 |
8.00 |
|
|
Type 2 |
(2-1) |
10 |
10 |
6 |
6 |
6 |
6 |
7.33 |
|
(2-2) |
10 |
10 |
10 |
0 |
6 |
0 |
6.00 |
|
|
Type 3 |
(3-1) |
8 |
10 |
6 |
10 |
4 |
4 |
7.00 |
|
(3-2) |
8 |
10 |
10 |
10 |
4 |
4 |
7.67 |
|
|
Type 4 |
(4-1) |
7.5 |
10 |
7.5 |
7.5 |
7.5 |
3.75 |
7.29 |
|
Type 5 |
(4-2) |
10 |
0 |
10 |
0 |
0 |
0 |
3.33 |
|
Type 6 |
(5-1) |
10 |
10 |
10 |
10 |
10 |
10 |
10.00 |
|
(5-2) |
8 |
10 |
10 |
8 |
8 |
8 |
8.67 |
|
|
Total |
91.5 |
90.00 |
89.50 |
67.5 |
61.50 |
49.75 |
74.96 |
|
Type |
1 |
2 |
3 |
4 |
5 |
6 |
| High Performance |
100.00 |
93.33 |
86.67 |
83.33 |
66.67 |
96.67 |
|
(0.00) |
(16.33) |
(16.33) |
(14.43) |
(57.74) |
(8.16) |
|
| Low Performance |
76.67 |
40.00 |
60.00 |
62.50 |
0.00 |
90.00 |
|
(19.66) |
(30.98) |
(30.98) |
(21.65) |
(0.00) |
(10.95) |
|
| Differences |
23.33 |
53.33 |
26.67 |
20.83 |
66.67 |
6.67 |
|
(21.29) |
(54.16) |
(35.02) |
(50.17) |
(57.74) |
(14.26) |
|
| Scores |
88.33 |
66.67 |
73.33 |
72.92 |
33.33 |
93.33 |
|
(18.01) |
(20.60) |
(27.41) |
(20.03) |
(51.64) |
(9.85) |
(1). Successful subjects used forward strategy more frequently, and the process of problem-solving was clear-cut. The answer sheet of Case Hf-2 in Type 1 is as follows:
(2). Considering the relative positions of a kind of ligand in a structure which it contained two kinds of ligands. This can be illustrated by Hf-1's prism shape prototype strategy as follows.
(3). Sometimes High Performance subjects also used the trial and error strategy, but belonging to the "systematic trial and error" strategy, and used checks to expel repeated isomers.
|
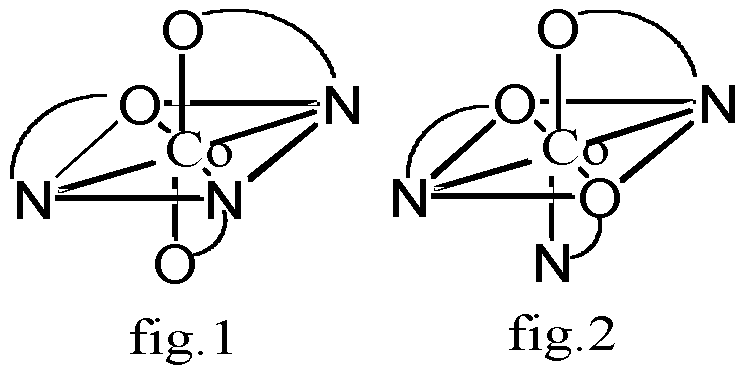
Hh-3: (octahedral) if 2 of 3 "a"s in opposition, then arbitrarily put the 3rd "a" anywhere in the square planar (Fig.1), if the opposite position is "b" instead of "a", then place 2 other "a"s on the opposite position of the square planar (Fig.2)....if the opposite position is still a,b, then the other 2"a"s are placed in the adjacent positions (Fig.3)... in such case to see if superimposable or not?actually, the first two are the same, because both 2"a"s are through the center of atom.
|
(1). Used worse reasoning strategy, and the change of problem-solving process were not clear, the answers were often incomplete or incorrect.
|
Ls-2. C is a planar hexagonal ...it could be 3 adjacent "a"s (fig.1)... or 2 adjacent "a"s, the other "a" staggered... the staggered could be every one (fig.2) or every two (fig.3)...actually only 2 isomers exist since fig.2 and fig.3 are the same.
|
(2). Used less effective trial and error and random process strategies, that experts considered to be the worse strategies.
|
Ls-1 |
(3). Used a more complex method when two or more were available. For example, when two ligands existed in a compound, they considered the complex one or two at the same time.
|
Ls-2:(octahedral as below)....if up and down are "a"s...any position of square planar is the same by free rotation clockwise or anticlockwise (fig.1)......if up and down are "b"s ...one case...up is an "a", down is a "b" (fig.2)...the other 2"a"s. "b"s are two cases,...one is adjacent (fig.3), the other is staggered (fig.4).
|
The number of times the strategies were used by solvers is shown in Table 5. High performance solvers used "Forward" and "Considering Simple Case" strategies more frequently than low performance solvers; and low performance solvers used "trial-and error" more frequently than high performance. We also discovered that low performance solvers had used "Random Trial and Error", "Analogy" and "Random Process" strategies none of which were used by in high performance solvers.
Table 5 The Strategies Used by Solvers
| Strategy |
Hf-1 |
Hf-2 |
Hh-3 |
Ls-1 |
Ls-2 |
Lf-3 |
Successful subjects |
Unsuccessful subjects |
| Forward |
8 |
9 |
7 |
4 |
4 |
2 |
24 |
10 |
| Systematic trial and error |
1 |
0 |
2 |
3 |
1 |
1 |
3 |
5 |
| Random Trial and Error |
0 |
0 |
0 |
1 |
3 |
0 |
0 |
4 |
| Considering Simple Case |
3 |
2 |
3 |
2 |
1 |
0 |
8 |
3 |
| Random Process |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
2 |
| Means- end |
0 |
0 |
1 |
1 |
0 |
2 |
1 |
3 |
| Analogy |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
High performance solvers had correct recognition of "geometric isomers" and "optical isomers", but low performance solvers often had wrong recognition for geometric isomers, and regarded the structures that can not overlap as geometric isomers; so sometimes they regarded optical isomers as geometric isomers. They regarded mirror images as optical isomers. During the process of solving problems, low performance solvers asked many questions because they did not understand the meaning of the problems, such as "optical isomers", "mirror image", "overlap", "ortho-position", "Ma2b2, Ma3b3", "reflection operator", "inversion operator", especially Lf-3 who asked 15 questions. All the low performance solvers and Hf-2 had misunderstanding of the "inversion operator", and they all had used "rotation operator" during the visualizing. According to the definition of the solvers on the inversion operator, low performance made solves many mistakes during the operation.
 Top
Top

 Header
Header
