E-Mail: rholling@metz.une.edu.au
Abstract: What do we mean by problem solving in chemistry? How can we teach problem solving most effectively? What role can computers play in teaching problem solving? There is a vast literature on problem solving in the sciences, which is a largely untapped resource. At the introductory levels, typical problems are usually routine applications of formulae rather than real-life, ill-structured or ill-defined problems. It is usually assumed that students will reach conceptual understanding just through sufficient practice it problem solving. Research indicates that it is the strategic use, rather than the mere possession of knowledge that improves understanding and learning. Instructional programs in problem solving, which make processes explicit and, which teach a range of strategies, have shown to be moderately successful at the very least. Students need to be made aware of the benefits of strategies, see them modelled and have the opportunity to practice them over a period of time to develop their problem solving skills. They can benefit by being given the opportunity to develop their metacognitive awareness and skills. Ways in which computers and ICT are being used in chemistry problem solving are reviewed and details are presented of an on-line tutorial we have developed for enhancing the problem solving skills of first year science students.
This paper focuses on the teaching of problem solving in chemistry at the first year university level. The subject of problem solving covers a very wide area and we can only touch on a number of points rather briefly here. These points include: What do we mean by problem solving in chemistry? In general terms how would we characterise past and current practice in teaching problem solving in chemistry? How should we teach problem solving effectively? What role can computers play in teaching problem solving?
There is a vast literature on problem solving in the sciences, which is a largely untapped resource. (Gabel, 1994) Traditionally, problem solving has been presented by lecturers and tutors doing problems and then setting similar problems for students to practise. At the introductory levels, typical problems are usually routine applications of formulas rather than real-life, ill-structured or ill-defined problems. It is usually assumed that students will reach conceptual understanding just through sufficient practice at problem solving. (Hobden, 1998) At best, by repetitive practice at problem solving, many students may gain routine expertise, but not adaptive expertise. That is, they may develop speed and accuracy at routine problem solving, but fail to develop the ability to reflect on what they do or to adapt to solving new problems in a flexible manner.
Research indicates that it is the strategic use, rather than the mere possession of knowledge that improves understanding and learning. Instructional programs in problem solving, which make processes explicit and which teach a range of strategies, have been shown to have some success. Students need to be made aware of the benefits of strategies, see them modeled and have the opportunity to practice them over a period of time to develop their problem solving skills. They can benefit by the opportunity to develop their metacognitive awareness and skills. Ways in which computers and ICT are being used in chemistry problem solving are reviewed and details are presented of an on-line tutorial we have developed for enhancing the problem solving skills of first year science students at the University of New England. (Hollingworth & McLoughlin, 2001)
“From the first days of science instruction, sets of routine problem tasks assigned by the teacher have been part of classroom life. As a teaching strategy, they have largely been used uncritically. It would appear that nearly all physical science education, and especially the physics component, seems to be based on the optimistic assumption that success with numerical problems breeds an implicit conceptual understanding of science.”
This quote from (Hobden, 1998) indicates what has largely been the practice in teaching problem solving in chemistry in the past and what sometimes continues into the present. Highlighting the quote here is not necessarily to negate the use of practice as a strategy for teaching problem solving, but to point out the lack of critical and informed thought, which often leads to the sole use of this method for teaching problem solving. (Gabel & Bunce, 1994) present a review of the literature on problem solving in chemistry. As chemists, we need to inform our practice as fully as possible by reference to educational research.
What exactly do we mean by problem solving in chemistry? Put in the most straightforward manner, problem solving is "What you do, when you don’t know what to do." (Wheatley, 1984) The definition of what is a problem thus depends on who is solving the problem. The level of expertise of the solver will determine whether a particular example is just an exercise or rather a real problem to them. Probing into what we may be teaching our students in problem solving, it becomes clear that we need to distinguish between routine exercises and novel problems. (Bodner, 1991) suggests that it is familiarity, rather than difficulty or complexity, that distinguishes an exercise from a problem. Exercises can often be solved by an algorithm and by forward reasoning. In contrast, novel problems may require an "anarchistic," cyclic and reflective approach for their solution.
(Johnstone, 1998) has proposed a useful classification of problem types as shown in Table 1.
| Type | Data | Methods | Outcomes/Goals | Skills Bonus |
| 1 | Given | Familiar | Given | Recall of algorithms. |
| 2 | Given | Unfamiliar | Given | Looking for parallels to known methods. |
| 3 | Incomplete | Familiar | Given | Analysis of problems to decide what further data are required. |
| 4 | Incomplete | Unfamiliar | Given | Weighing up possible methods and then deciding on data required |
| 5 | Given | Familiar | Open | Decision making about appropriate goals. Exploration of knowledge networks. |
| 6 | Given | Unfamiliar | Open | Decisions about goals and choices of appropriate methods. Exploration of knowledge and technique networks |
| 7 | Incomplete | Familiar | Open | Once goals have been specified by the students the data are seen to be incomplete |
| 8 | Incomplete | Unfamiliar | Open | Suggestions of goals and methods to get there, consequent need for additional data. All of the above skills. |
Table 1 Johnstone's classification
of problems
We need to examine carefully what types of exercises and problems
we are presenting our students. Are we selling them short by only
giving them exercises of the closed type in Johnstone's classification?
If we want our students to become expert problem solvers and to
develop higher-level skills, then we need to help them to progress
beyond the skills to solve routine exercises. We need to consider
how much of what we do with our students is working on routine
exercises and how much is on novel problems, and what should be
the balance between these. We may be doing a good job at teaching
our students how to solve exercises, but are we doing as good
a job in teaching them how to solve real problems?
Moreover, what sort of expertise are we helping them develop?
Through repetitive practice working on exercises many students
will gain routine expertise, but not adaptive expertise. (Hatano & Inagaki, 1986)
That is, they may develop speed and accuracy at routine exercise
solving, but fail to develop the ability to reflect on what they
do or to adapt to solving new problems in a flexible manner.
There is a changing emphasis on problem solving occurring in the
standard chemistry textbooks. Most texts now include clearly delineated
types of problems at the end of the chapter. These include drilling
exercises, conceptual problems and more challenging problems.
For example see (Kotz &
Treichel Jr, 1999), (Moore,
Stanitski, & Jurs, 2002), (Brady,
Russell, & Holum, 2000). Within the bodies of newer editions
of the texts, there are explicit discussions of problem solving
processes. There are problem solving toolkits, and problem solving
checking and monitoring hints. There is also now a growing list
of books devoted entirely to developing students thinking skills.
See for example, (Barouch,
1997), (Bucat & Shand,
1996), (Garratt, Overton,
& Threlfall, 1999).
Increasingly texts are now accompanied by CD ROMs, which may include
a variety of problems, test banks and other learning resources,
which involve active participation. For example, Saunders General
Chemistry CD ROM. (Kotz &
Vining, 1999) The textbook publishers also now provide websites
devoted to specific texts. Many of these are open only to those
who have purchased the text, but there is limited access to a
number of sites, which include problem test banks. For example,
the Companion
Website to Chemistry for Changing Times. (Hill
& Kolb, 2001)
The question of how we should teach problem solving in chemistry is a difficult one to answer. We have already mentioned some points about past and current practice and considered the different types of exercises or real problems we may pose to our students. How then can we help them most effectively to become expert problem solvers? Certainly practice is essential, but it needs to be the right kind of practice. (Reif, 1995)
“Adequate practice is certainly needed to learn problem solving. But it must be the right kind of practice! In athletics or music instruction, the wrong kind of practice cannot only be ineffective, but harmful (leading to poor habits which are difficult to break or even to injuries). Similarly when students working on homework problems spend hours floundering, what they really practice is floundering. And if they spend most of their time haphazardly grabbing miscellaneous equations, they certainly do not practice valuable problem solving skills."
How useful are the worked examples that students see in their textbooks or which we may provide on the board in a tutorial? (Herron, 1990) warns that the worked examples in textbooks give students an unrealistic idea of how problems are actually solved.
"The examples provide no indication of the false starts, dead ends, and illogical attempts that characterise problem solving in its early stages, nor do they reveal the substantial time and effort expended to construct a useful representation of a problem before the systematic solution shown in examples is possible."
This highlights the need to examine carefully what we are doing about teaching the actual processes of problem solving. Students are rarely given an insight into what actually occurs in the mind of an expert when they tackle either an exercise or a real problem.
Whatever approach we may take to assist our students develop their problem solving skills, whether it is through personal teaching or through the use of computer technology, our aim is to guide them along the transition from novice to expert. Pattern recognition and the ability to identify the deep underlying principles relevant to a problem are what distinguish expert performance in problem solving. (Bransford, Brown, & Cocking, 2000) list several key principles of experts' knowledge, which can help guide where we might concentrate our attention as we develop our own approach to teaching problem solving. These include:
Expertise is only built up over a long period of study, but
it will be most beneficial to students to become aware of these
factors as they progress towards expertise. As experts we need
to be able to communicate explicitly about our own expertise,
to give examples of how this affects our problem solving, to help
students acquire more effective problem solving skills. Unfortunately
expertise in a particular subject area does not guarantee that
one will also be an expert teacher in that area. Skills and processes
may have become so automatised that the expert may find it difficult
to appreciate the difficulties a novice student faces.
What happens when the expert tackles, what for them is an exercise,
is very different to what happens when the students tackles the
same task, which for them may well be a problem. Experts are able
to see the deep underlying principles behind the problem and use
their well-connected knowledge base to proceed to a solution.
Despite this, there may be benefit to students to become more
aware of these processes, if the experts themselves can become
aware of the processes they use and be able to describe them explicitly.
If students were made aware of what happens when experts tackle
real problems, they may well find that this is closer to their
own problem solving processes. They could benefit greatly from
learning about the important monitoring and control processes
the experts use in guiding themselves to a solution.
A considerable amount of research has been done into the effectiveness of different approaches to teaching problem solving in chemistry in the face-to-face situation. Many of the studies have been at primary and high school level, while less work has been done at the university level. (Taconis, Ferguson-Hessler, & Broekkamp, 2001) have reviewed experimental work in teaching problem solving in science over the years 1985 - 1995 and which has been reported in high quality journals. The characteristics of effective treatments were found to be:
The experimental works reviewed were those adopting a cognitive approach, because metacognitive aspects were not made explicit in many of the studies reviewed. It is clear that whatever approach is taken, the interventions must be long term in order to produce any significant improvement in problem solving. Even though the review looked at experimental work involving personal teaching situations, it is clear that teaching problem solving in a computer environment is ideally suited to addressing the above guidelines. In fact one of the strongest advantages of computers and one most appreciated by students, is the ability to provide immediate feedback.
The term metacognition refers to a learner's knowledge about
their own processes of cognition and the ability to control and
monitor those processes as a function of the feedback they receive
through the outcomes of their learning. (Metcalfe
& Shimamura, 1994) Metacognitive approaches to developing
problem solving skills need a long-term intervention, but such
programs, which make processes explicit and which teach a range
of strategies, have been shown to produce some success. If students
realise that they already have some problem solving skills and
they are explicitly taught problem solving skills in the context
of disciplinary knowledge, and encouraged to develop metacognitive
awareness of these skills, the result should be, a speeding up
of the learning process, new information becoming easier to learn,
and an enhancement of overall academic performance.
(Gredler, 1997) proposed
three essential instructional conditions for any development of
metacognitive skills.
Similar conditions were proposed by (Masui & De Corte, 1999), who suggested an integrated set of instructional principles for an effective learning environment to enhance learning and problem solving skills for university students. These are:
The above two sets of guidelines are presented for metacognitive approaches to problem solving in general. Considering explicitly the use of computer technology in the teaching of problem solving, (Lin, Hmelo, Kinzer, & Secules, 1999) suggested the following are essential factors in designing technology to support reflection.
By process displays it is meant that the student’s own problem solving processes are displayed to them in some form of playback. Process prompting and modeling help students to become more aware of the skills required and to gradually build up their own skills. Reflective social discourse can occur when students are given the opportunity to discuss their learning activities in a supportive community of learners.
Searches of the Internet on such queries as, "problem
solving in chemistry, "teaching chemistry problem solving,
"on-line problem solving," etc, result in several hundred
hits. Many of these hits are to university chemistry unit home
pages where problem solving is mentioned as part of the curriculum.
Unfortunately, as more learning materials are developed for the
on-line environment, they are being placed in Webshells, such
as WebCT or Blackboard, where only students enrolled in subjects
have access to the materials. This means we are not able to readily
access on-line sites, where others may well be making progress
in teaching problem solving effectively. There are very few accessible
sites, which explicitly address the teaching and learning of chemistry
problem solving.
There are many sites at universities and publishers, either accessible
or protected by passwords, which contain question databases. Many
of these contain multiple choice questions and have the benefit
of immediate feedback, but concentrate on recall of facts, simple
algorithms, and closed exercises, rather than higher level problems.
The commercial CD ROMs provide a wider range of learning activities
to work on problems in different modes and to test qualitative
understanding, but still lack in tackling real problem solving.
Below we note several sites, which do consider problem solving
explicitly.
Chemistry problem solving websites
The A to E Approach

This is a well known website which gives a detailed heuristic for problem solving. It is a very useful resource for teaching chemistry problem solving. There is some emphasis on metacognitive skills in so far as the heuristic suggests students reflect on the process of the problem solution, monitor what they are doing and evaluate their answer. Unfortunately there are no worked examples, which could illustrate these points specifically.
21st Century Problem Solving
This site, which includes problem solving for a number of science subjects, presents another heuristic. It does give a very few specific examples, but there is no explicit discussion or illustration of what cognitive and metacognitive skills are needed in determining solutions.
Purdue University
http://chemed.chem.purdue.edu/genchem/probsolv/index.html
The Purdue University General Chemistry website does give some worked examples. Problems are presented with a strategy for solution and students can follow through the solution step by step. The limitation is that there are only a fairly small number of problems on basic chemistry topics and the problems are closed exercises. In this regard they correspond to Johnstone's Type 1 problems and will not develop students' problem solving skills to a high level.
http://cw.prenhall.com/bookbind/pubbooks/hillkolb/
The Prentice Hall website for the text, Chemistry for Changing
Times, by John W. Hill and Doris K. Kolb gives an excellent example
of the type of resources now connected on-line to a textbook.
This textbook is aimed at non-major students of chemistry. At
the website there is a Media Lab, Web References and On-line Projects
as well as Practice Questions. The activities involved in the
Media Lab and On-line Projects do require higher order cognitive
skills and should be engaging to students in their learning of
chemistry.
The questions in the Practice Question databank are multiple choice
type questions, mainly involving recall of facts and application
of algorithms. The benefits of the site are that students may
look at a hint in answering questions and then, when a test is
submitted immediate feedback is given. The feedback includes further
information about the incorrect answers the student has given,
as well as an explanation of the correct answer.
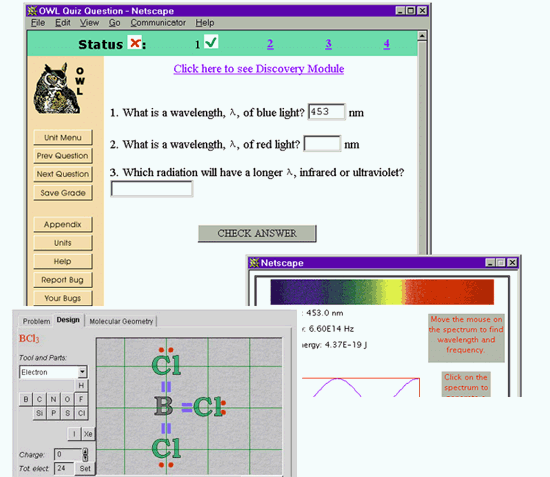
OWL
http://ccbit.cs.umass.edu/owl/
OWL (Online Web-based Learning) is an electronic learning environment originally developed at the University of Massachusetts for General Chemistry and since extended to other disciplines. (Hart, 2000) Its scope of electronic homework delivery extends from straightforward electronic quizzing to richer interactive learning environments, including guided discovery exercises and intelligent tutors. While the emphasis is on learning chemistry and providing an electronic homework environment, the site is mentioned here because the introduction of intelligent tutors hints at future developments for teaching chemistry problem solving. The needs of the individual students are addressed by customizing instructional strategies with intelligent tutors. These computer tutors vary the pace of instruction and present problems at the appropriate level to challenge each individual student. Access to a very limited demonstration site is available on request. Some partial screen dumps are shown in the figure below.
At the University of New England we have built an on-line tutorial
aimed at developing the problem solving skills of first year science
students. The tutorial actually covers problem solving in the
subject areas of Biophysics and Biology, as well as Chemistry.
By preference we would teach problem solving in a face-to-face
situation, where student-teacher and student-student interactions
could be maximised, but, since external students represent a significant
proportion of our students, it was important for us to develop
some tutorial assistance for these students, which led us to an
on-line tutorial. (Hollingworth
& McLoughlin, 2001)
Our internal and external students represent two more or less
separate groups; while there are some similarities, there are
also obvious differences. Internal students by and large are recent
school leavers and tend to be younger. Overall they tend to be
less motivated and in many cases do not have a clear idea of their
long-term goals. Some may be enrolled in units not of their liking,
but they persist because these units may be pre-requisites for
a particular course of study. External students on the other hand
are usually more mature students coming back to part-time study
after a period in the workforce. They are usually highly motivated,
with more clearly defined goals for their studies. Some externals,
who have been at study for some time, are likely to be more reflective
learners, aware of their own strengths in learning, but may have
experienced little formal training in developing meta-learning
strategies in their studies in science.
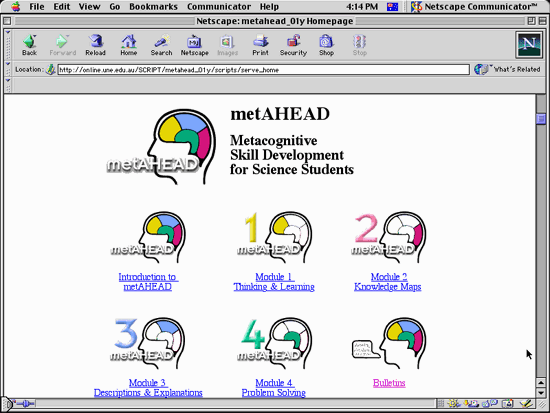
The home page of the metAHEAD tutorial is shown in the accompanying figure. The tutorial consists of several modules.
On first use and again at the end of the semester, students
do a short metacognitive inventory questionnaire. This allows
them to track their development over the longer term. The first
module of the tutorial then introduces students to the concepts
of cognition and metacognition, as well as helping them explore
a little about their own thinking processes and problem solving
skills. Module 2 introduces knowledge maps, a useful metacognitive
tool for organizing the students' knowledge.
Modules 3 and 4 are similar in structure and deal with the two
broad classifications of problems, those involving descriptions
or explanations of science phenomena, and quantitative problems.
Unfortunately the tutorial is built in the WebCT environment and
is only accessible to students enrolled in our chemistry units.
A number sample pages give some idea of the content
of the tutorial.
The accompanying flow chart illustrates
in detail the module on quantitative problem solving in science
showing the parallel paths for each subject area and topics within
each subject area. Having decided on a subject and topic, the
student is first faced with a brief test of their knowledge on
that topic. The tutorial does not aim to develop content knowledge,
but rather metacognitive skills. Thus, there is a point in students
proceeding with the tutorial and starting to solve some problems,
only if they have sufficient content knowledge.
The choice of problems for the tutorial was very important. On
the one hand we wished to include exercises or problems like those
which students work on in the usual assignments they are given
when studying a subject. Experience suggests that the very students
we wish to target may not be interested in the tutorial unless
they can see a very direct relevance to their usual work in the
subject. (Note that we personally have little control over the
selection of these problems.) On the other hand, if we wish to
develop our students’ metacognitive skills, then we need
to go beyond merely drill type exercises.
Johnstone’s classification of problem types in Table
1 makes the reason for this clear. Type 1 problems, where
all the data is given, the method of solution is familiar and
there is one clear given outcome basically only requires the recall
and application of algorithms. Some metacognitive ability is required
to monitor and control the process of solution and to check on
the answer, but this is not very great when the students is merely
following a well defined path to solution of the problem. As we
move to higher-level problems more monitoring is required. For
example, in a Type 3 problem, with incomplete data the students
will need to decide what data is required and where to find it,
as well as determine when they have all the requisite data. In
a Type 4 problem, where the method is unfamiliar, the students
will need more well developed analytical and monitoring skills
to test alternative methods as necessary and then check that their
chosen method is leading towards a solution.
Problem Types 5 to 8 represent more ill defined problems, where
the outcome or goals are open. There may not be just one clear
straightforward answer to the problem. The student’s knowledge
of their own knowledge is more clearly tested as they make decisions
about solutions and choices of appropriate methods towards solutions.
A more thorough exploration of their own knowledge and technique
networks will be required as they grapple towards a solution.
These are the sorts of problems professionals face in the real
world in their everyday jobs, where goals are not very clearly
defined, where at least initially there is not only one solution,
where the data required for a solution must be decided upon and
then collected and where there is not necessarily a routine method
for solution. If we wish to prepare our students to function effectively
in their workplace, then we need to gradually build up their skills
by exposing them to these types of problem.
In each module and subject the student progresses from drill type
exercises to more ill defined, real-world problems involving higher-level
cognitive abilities. The aim is to bring the student to a better
awareness of the processes they should use and to model more effective
processes when appropriate. Starting with simpler problems the
student is given assistance with planning a solution and the use
of strategies. Students are encouraged to write their answers
and reflective comments for each problem in their own on-line
logbook. Reflection on what they have learnt from particular exercises
can be beneficial, if it does not just focus on the solution,
but the thinking and planning processes of problem solving.
After working through a problem the student has the opportunity
to view other students' answers, both good and poor, and the lecturer's
model answer. Comments are supplied on these answers together
with "metacomments," that is comments about metacognitive
aspects of the answers. In some cases there are also audio or
video clips of students describing their own working of the problem,
as shown in the figure below. (See also Sample
Pages.)
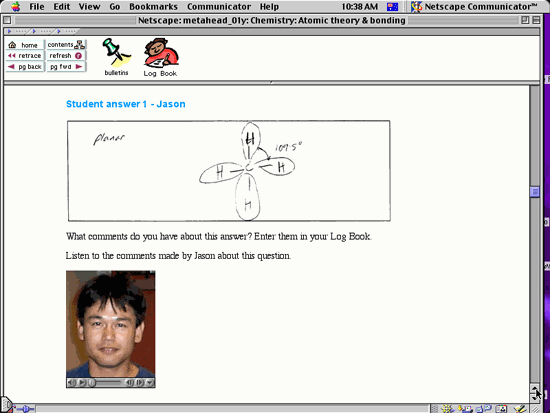
Depending on their satisfaction with their solution to a problem,
the student may elect to try another very similar problem or move
onto another problem type. As students progress through the topic
the problems become more complex requiring higher order skills
and use of strategies. More emphasis is placed on discussion of
strategies and reflection on processes on the bulletin board at
this stage. Small group discussion with peer feedback also helps
students to consider perspectives other than their own.
Throughout the tutorial the emphasis is on process and on student
reflection. Reflective thinking requires the student to organise,
monitor and evaluate their thinking and learning to come to a
deeper understanding of their own processes of learning. Our design
of this technology-based environment builds in important implications
of constructivist theory. As the development of metacognitive
skills occurs over the long term, it is essential that this progress
is recorded in the student's own on-line logbook and in the bulletin
board discussions, both of which are available to the student
for reference over the semester.

Student feedback has been obtained both during the development phase and at the end of the first phase of building the tutorial. No formal or rigorous evaluation of the tutorial has been carried out yet, but this is planned for the future. Some student comments below give a sense of the reaction to the tutorial.
At present we have only included one or two topics for each
subject in each module of metAHEAD. In the future we aim to add
more topics, which will give students a choice of topics to work
on for developing their problem solving skills in each semester.
We would also like to add more audio and video clips of students
talking about aspects of their problem solving. These will help
illustrate to students both the benefits of effective strategies
and well developed metacognitive skills, as well as the deficiencies
of poor skills. Together with these additional audio and video
clips we aim to expand the range of "metacomments" that
accompany the students' answers and the lecturers' model answers.
These will then bring to our students' awareness a wider range
of effective strategies and metacognitive skills they can apply
in their problem solving in chemistry. They will also highlight
the many pitfalls students can encounter when problem solving
and ways to overcome them.
To date some preliminary feedback from students has been obtained,
while developing the tutorial and upon completion of the first
stage, as evidenced by the student comments above. A rigorous
educational evaluation of the tutorial is planned for next year.
We have given a very brief outline in the above of some different
ways in which the teaching chemistry problem solving is being
approached using computers. Teaching problem solving in chemistry
effectively is no easy task even in a face-to-face situation with
our students. Many additional difficulties must be faced in trying
to provide our students with effective resources to develop their
problem solving skills in a computerised learning environment.
Some of these difficulties are being faced and overcome as computers
become more powerful and computerised learning environments become
more sophisticated.
A major difficulty in the computer environment is in providing
very specific feedback to each step of the solution of a problem,
which a student is attempting. Computer analysis of student solutions
presents major difficulties, because there is no unique correct
method of solution, even for the simplest chemistry problems.
This can be done easily in the face-to-face situation with an
expert on hand. In the computer environment a system of appropriate
feedback can be built up with considerable effort for simple closed
exercises, but this is impractical when it comes to more complex
or ill-defined problems. Moreover, if students are to develop
adaptive expertise they must be exposed to a wide variety of problems.
We suggest that, in the future, as these computer learning resources
for problem solving are developed, we should bear in mind the
following points:
Barouch, D. H. (1997). Voyages in Conceptual Chemistry. Sudbury, MA: Jones & Bartlett Publishers.
Bodner, G. M. (1991). A View from Chemistry. In M. U. Smith (Ed.), Toward a Unified Theory of Problem Solving (pp. 21-33). Hillsdale, NJ: L. Erlbaum Associates.
Brady, J. E., Russell, J. W., & Holum, J. R. (2000). Chemistry: Matter and its Changes (Third ed.). New York: John Wiley and Sons.
Bransford, J. D., Brown, A. L., & Cocking, R. R. (2000). How People Learn: Brain, Mind, Experience, and School. Expanded Edition. Washington, DC: National Academy Press.
Bucat, B., & Shand, T. (1996). Thinking Tasks in Chemistry, Teaching for Understanding. Perth: University of Western Australia.
Gabel, D. (Ed.). (1994). Handbook of Research on Science Teaching and Learning. New York: Macmillan Publishing Company.
Gabel, D. L., & Bunce, D. M. (1994). Research on Problem Solving: Chemistry. In D. L. Gabel (Ed.), Handbook of Research on Science Teaching and Learning (pp. 301-326). New York: Macmillan Publishing Company.
Garratt, J., Overton, T., & Threlfall, T. (1999). A Question of Chemistry: Creative Problems for Critical Thinkers. Harlow: Longman, Pearson Education Limited.
Gredler, M. E. (1997). Learning and Instruction, Theory into Practice. NJ: Merrill, Prentice Hall.
Hart, D. (2000). The Online web-based Learning (OWL) Software System. Paper presented at the Syllabus 2000.
Hatano, G., & Inagaki, K. (1986). Two courses of expertise. In H. A. H. Stevenson, & Hakuta, K. (Ed.), Child development and education in Japan (pp. 262-272). New York: Freeman.
Herron, J. D. (1990). Research in chemical education: Results and directions. In M. Gardner & G. J. Greeno & F. Reif & A. H. Schoenfeld & A. A. diSessa & E. Stage (Eds.), Toward a Scientific Practice of Science Education (pp. 31-54). Hilldale, NJ: L. Erlbaum Associates.
Hill, J. W., & Kolb, D. K. (2001). Chemistry for Changing Times (Ninth ed.). Upper Saddle River, NJ: Prentice Hall.
Hobden, P. (1998). The role of routine problem tasks in science teaching. In B. J. Fraser & K. G. Tobin (Eds.), International Handbook of Science Education (Vol. Part 1, pp. 219-231). Dordrecht: Kluwer Academic Publishers.
Hollingworth, R. W., & McLoughlin, C. (2001). Developing
science students metacognitive problem-solving skills online.
Australian Journal of Educational Technology, 17, 50-63.
http://cleo.murdoch.edu.au/ajet/ajet17/hollingworth.html
Johnstone, A. H. (1998). Learning through problem solving. In D. Rafferty & S. Sleigh (Eds.), Problem Solving in Analytical Chemistry (pp. v-viii). London: The Royal Society of Chemistry.
Kotz, J. C., & Treichel Jr, P. (1999). Chemistry and Chemical Reactivity (Fourth ed.). Fort Worth: Saunders College Publishing.
Kotz, J. C., & Vining, W. J. (1999). Saunders Interactive General Chemistry CD-ROM (Version 2.5). Fort Worth: Saunders College Publishing.
Lin, X., Hmelo, C., Kinzer, C. K., & Secules, T. J. (1999). Designing Technology to Support Refelection. Educational Technology Research and Development, 47(3), 43-62.
Masui & De Corte (1999). Enhancing Learning and Problems Solving Skills: Orienting and Self-Judging, Two Powerful and Trainable Learning Tools. Learning and Instruction, 9(6), 517-542.
Metcalfe, J., & Shimamura, A. P. (1994). Metacognition: Knowing about Knowing. Cambridge, MA: MIT Press.
Moore, J. W., Stanitski, C. L., & Jurs, P. C. (2002). Chemistry: the Molecular Science (First ed.). Forth Worth: Harcourt College Publishers.
Reif, F. (1995). Millikan Lecture 1994: Understanding and teaching important scientific thought processes. American Journal of Physics, 63(1), 17-32.
Taconis, R., Ferguson-Hessler, M. G. M., & Broekkamp, H. (2001). Teaching Science Problem Solving: An Overview of Experimental Work. Journal of Research in Science Teaching, 38(4), 442-468.
Wheatley, G. H. (1984). Problem solving in school mathematics (MEPS Technical Report No. 84.01). West Lafayette, IN: Purdue University, School of Mathematics and Science Center.
 Top
Top

 Header
Header
