Chemical Education Journal (CEJ), Vol. 6, No. 2 /Registration No. 6-11/Received November 9, 2002.
URL = http://www.juen.ac.jp/scien/cssj/cejrnlE.html
SETTING UP THE CD-ROM ON: " VIRTUAL EXPERIMENTS AND CHEMICAL EXPERIMENTS FOR 10-TH CLASS IN VIETNAM "
Dr. Nguyen Duc Chuy
Faculty of Chemistry, Hanoi University of Education of Vietnam, Vietnam
E-mail: nttchuy@yahoo.com
Abstract
This paper deals with an important problem in Vietnam related to the teaching of chemistry. This is the construction of software containing virtual and "hands-on" chemistry experiments, which have been presented as the Web pages on a CD-ROM. The CD- ROM "Virtual experiments and chemistry experiments for 10-th class in upper secondary school", is easy to use, and has many advantages.
After 15 years of renovation, Vietnam continues its development. Nowadays to implement the strategy of the revolution, " industrialization and modernization," Vietnamese Education in general and Chemical Education in particular must be renewed.
One of the important steps for the renewing of teaching and learning is equipment, which for Chemistry means laboratory equipment for chemical experiments.
Vietnam is still a poor country and there are still not enough resources for provision of equipment to lower secondary school and upper secondary school. For that reason, chemistry has involved teaching and learning without chemical
experiments in rural secondary schools except some schools in large towns - Hanoi and Ho Chi Minh cities. Besides, Vietnam is a tropical country with a hot and humid climate, it is impossible to preserve chemical substances in the secondary school. There are complicated and toxic chemical experiments, which are impossible
to carry out in secondary schools. Furthermore, secondary school teachers do not have enough time to prepare chemical experiment demonstrations. Therefore we must rely on information and communication technology.
First of all we set up a CD-ROM " Virtual Experiments and Chemical Experiments for 10-th class in Vietnam Upper Secondary Schools". Virtual experiments are used to explain abstract concepts about atomic structure, atomic orbitals, chemical bonds, overlapping of atomic orbitals and so on... Movies
of chemical experiments are used as source materials to present chemical experiments in the class.
The CD-ROM has been set up in the form of Web pages.
At the left column of the Web page, there are Contents of the CD (see picture 1), and at the right the principal Web page, explains the content of the virtual experiment or describes the procedure to carry out the chemical experiment and a click on " Xem phim " will display the movie animation and narration
in the Vietnamese language.
Contents
Chapter 1. Atomic Structure
I Generalities
I.1. Experimental data for the complete structure of atom: Electron, Nucleon, Proton, Neutron.
I.2. Summary of atomic structure.
II. Structure: Dimension and mass of atom.
II.1. Structure
II.1.1. Nucleon: Proton, Neutron, Nucleon charge, Mass number.
II.1.2. Electron
II.2. Dimension and mass
III. Chemical Elements: Definition, Atomic number, Symbol.
IV. Isotope. Mass Definition, average atomic mass.
V. Distribution of electrons in atoms: Electron motion in atoms, classes of electrons, subclasses of electrons, Orbital, maximum number of electrons in the class and subclass, electron configuration, electron character in each class.
VI. Presentation of some forms of atomic orbitals: Orbitals s, p, d, f.

Picture 1.
Chapter 2. The Periodic System of Chemical Elements.
I. Principle of element arrangement.
II. Presentation of a short form of the periodic table: Order number, period, group and subgroup.
III. Periodic law: definition, role of periodic table.
IV. Periodic properties of elements.
V. Presentation of some forms of Periodic tables.
Chapter 3: Chemical bonds
I. Why molecules form from atoms?
II. Valence bond
II.1. Forming of bonds in hydrogen molecule.
II.2. Forming of bond in hydrogen chloride (see picture 2)
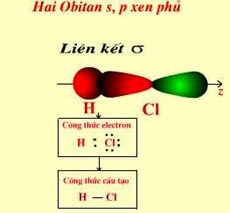
Picture 2. Overlap of orbital s and p
II.3. Forming of bonds in chlorine molecule.
II.4. Forming of bonds in nitrogen molecule.
III. Ionic bond
III.1. Formation of ions.
III.2 Formation of sodium chloride.
IV. Presentation of some crystal models: molecules, atomic and ionic crystals.
Chapter 4. Principal subgroup 7: Halogen group.
I. Chlorine.
I.1. Preparation of chlorine gas
I.2. Reaction of chlorine with aluminum.
I.3. Reaction of chlorine with iron (see picture 3)

Picture 3
I.4. Reaction of chlorine with copper.
I.5. Reaction of chlorine with hydrogen.
I.6. Change of color by humid chlorine
II. Hydrogen chloride.
II.1. Preparation of hydrogen chloride gas.
II.2. Reaction of hydrogen chloride gas with sodium hydroxide solution.
III. Hydrochloric acid and chloride salts.
III.1. Hydrochloric acid changes color of indicator
III.2. Reaction of hydrochloric acid with copper (II) oxide
III.3. Reaction of hydrochloric acid with copper (II) hydroxide.
III.4 Reaction of hydrochloric acid with iron (III) hydroxide.
III.5. Reaction of hydrochloric acid with sodium carbonate.
III.6. Reaction of sodium chloride with silver nitrate.
III.7. Reaction of hydrochloric acid with calcium carbonate.
III.8 Identification of sodium carbonate.
IV. Bromine, Iodine.
IV.1. Sublimation of iodine.
IV.2. Reaction of bromine with aluminum (see picture 4).

Picture 4
IV.3. Chlorine displaces bromine from its salt solution.
IV.4. Bromine displaces iodine from its salt solution
Chapter 5. Principal subgroup 6: Oxygen and sulfur.
I. Oxygen
I.1 Preparation of oxygen gas
I.2 Reaction of oxygen with iron.
I.3 Reaction of oxygen with sodium (see picture 5).

Picture 5
I.4 Reaction of oxygen with phosphorus
II. Sulfur.
II.1 Allotropy of sulfur
II.2 Reaction of sulfur with hydrogen.
II.3 Reaction of sulfur with oxygen.
II.4 Reaction of sulfur with iron.
II.5 Reaction of sulfur with zinc.
III. Hydrogen sulfide gas.
III.1 Preparation of hydrogen sulfide gas and its burning.
III.2 Identification of sulfide anion by cadmium nitrate solution.
III.3 Identification of sulfide anion by copper sulfate solution.
IV. Oxides of sulfur
IV. 1 Sulfur dioxide gas changes color of rose
IV. 2. Sulfur dioxide gas changes color of sodium permanganate solution.
V. Sulfuric acid.
V.1 Sulfuric acid changes color of litmus solution.
V.2 Concentrated sulfuric acid absorbs water, converts sugar to carbon
V.3 Reaction of sulfuric acid with iron (III) oxide.
V.4 Reaction of sulfuric acid with copper (II) oxide.
V.5 Reaction of sulfuric acid with copper (III) hydroxide.
V.6 Reaction of sulfuric acid with sodium hydroxide.
V.7 Reaction of sulfuric acid with iron.
V.8 Reaction of sulfuric acid with calcium carbonate.
V.9 Reaction of concentrated sulfuric acid with copper.
V.10. Identification of sulfuric acid.
Chapter 6. Theory of reaction rate.
I. Enthalpy of reaction (Heat of reaction).
II. Rate of reaction.
III. Chemical equilibrium.
The CD-ROM is easy to use and has many advantages as follows:
* Chemical experiments are presented successfully, with safety and in time for teaching.
* It can be used for pupils learning chemistry at home.
* Chemical experiments may be accessed easily and watched many times.
* It can bear the hot and humid climate of Vietnam.
* It is internationally standardized, and may be compatible for all PCs.
* The capacity of the CD-ROM is large, and can include all the chemical experiments for a chemistry course for a class.
Hanoi 06-02-2002
 Top
Top

 Abstract
Abstract

 CEJ Vol. 6, No. 2, Contents
CEJ Vol. 6, No. 2, Contents





