Chemical Education Journal (CEJ), Vol. 7, No. 2 /Registration No. 7-15/Received
October 10, 2003.
URL = http://www.juen.ac.jp/scien/cssj/cejrnlE.html
Microscale experiments for understanding
Christer Gruvberg
Gothenburg University, Department of Chemistry, SE-412 96 Gothenburg, Sweden
University of Halmstad, Section for Economics and Engineering, Box 823, SE-301 18 Halmstad, Sweden
Director of the Swedish Microscale Chemistry Center, Aprilv 14a, SE-302 40 Halmstad, Sweden
mail: gruvberg@kreativkemi.se
Presentation
Experiment 1
Experiment 2
Summary
Presentation:
Two experiments: "The Number of Water Molecules in a Salt"
and "Colorimetric Determination of a Copper Sulfate Solution"
used in sequence make a combination of great value for the student's
understanding of stoichiometrics. Both experiments are using cheap
and simple equipment.
The first experiment makes the student aware of the fact that
the blue color in the hydrated copper sulfate is dependent on
both the copper ions and the water; when water is evaporated from
the salt the color is vanishing as well; when water is added to
the anhydrous salt the blue color reappear.
The calculations on the ratio of water molecules per salt unit
are making the student familiar with the stoichiometric ideas.
As a bonus the manipulations demonstrate that heating breaks the
bonds to the water molecules and that heat is released when the
salt is hydrated again.
The second experiment demands logics combined with the recent
acquired experiences as well as a retrospect on the theory picked
up during the lectures.
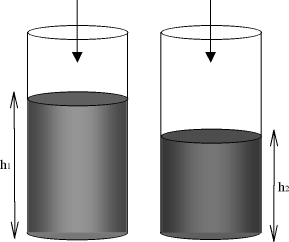 The later experiment contains a discovery experience
when the students find out that the color intensity (seen along
the length of the tube it is in, see the arrows in the tubes in
the figure below) is independent of diluting. The observed result
is surprising and it is challenging the students to understand
why. When the students are able to explain the phenomena as "same
color means same number of moles" (n1=n2)
then they are also able to convert the formula into c1V1=c2V2,
and because the tubes are identical the bottom areas are equal
which simplifies the formula to c1h1=c2h2
( n is number of moles, c is the mole concentration, V is the
volume and h is the distance through the liquid when the color
intensity was visually estimated). The instruction for preparing
the analyzed solution is given in g/L, the colorimetric result
is given in moles/L. When the grams per liter are converted into
moles/L the students see the relation between mass and moles.
Beside a lot of cognitive work they also gain a lot of manipulative
skills useful in most common laboratories.
The later experiment contains a discovery experience
when the students find out that the color intensity (seen along
the length of the tube it is in, see the arrows in the tubes in
the figure below) is independent of diluting. The observed result
is surprising and it is challenging the students to understand
why. When the students are able to explain the phenomena as "same
color means same number of moles" (n1=n2)
then they are also able to convert the formula into c1V1=c2V2,
and because the tubes are identical the bottom areas are equal
which simplifies the formula to c1h1=c2h2
( n is number of moles, c is the mole concentration, V is the
volume and h is the distance through the liquid when the color
intensity was visually estimated). The instruction for preparing
the analyzed solution is given in g/L, the colorimetric result
is given in moles/L. When the grams per liter are converted into
moles/L the students see the relation between mass and moles.
Beside a lot of cognitive work they also gain a lot of manipulative
skills useful in most common laboratories.
Experiment 1: "The Number of Water Molecules in a Salt".
Instrumentation: Cut off Pasteur pipettes, Blue fresh hydrated
copper sulfate salt (CuSO4 5H20), spatula, tripod, wire gauze, micro-torch and
mg-balance.
Procedure:
Cut off the Pasteur pipette at the spot marked
with the broken line. Weigh the empty pipette to the nearest mg
on the balance. Spread out some 0.3g blue salt. Weigh the pipette
with salt to the nearest mg. The difference between the two mass
values is the mass of blue salt. Place the wire netting on the
tripod and place the pipette on the netting. Heat the pipette
gently using the micro torch or any available burner. It is important
not to heat too hard in order to avoid sulfur dioxide release
from decomposing sulfate. It is also important to heat the whole
pipette to stop the escaping water molecules from condensing in
the pipette openings which can cause splattering and loss of salt.
Continue heating for another minute when the salt has lost its
blue color. Weigh the pipette when it has cooled for a few minutes.
The mass difference between the two latest weighings is the mass
of lost water. Estimate the ratio between the number of water
molecules and copper ions after the masses has been calculated
into moles. The expected number is five, in practice the value
is often closer to 5.2.
Experiment 2: "Colorimetric Determination of a Copper Sulfate Solution".
Comparation (comparing the color intensities seen
from the side)

|
Colorimetrics (comparing the color intensities
seen from above)
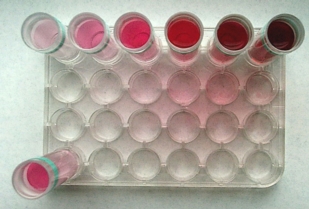
|
Instrumentation:
Two 12mL plastic colorimetric tubes, ruler, reference solution
(0.10M CuSO4), filter paper, 10 mL volumetric flask, weighing
boat, mg-balance.
Procedure:
Prepare a solution with 15.0 g
blue salt per liter in the 10 mL volumetric flask. To speed up
the dissolving of the salt, the solution can be warmed up by pouring
warm tap water on the outside of the flask.
Fill the two colorimetric tubes to one third of its volume (the
same volume in both tubes) with a colored solution (red food coloring
is recommended). Predict what the intensity of the colors in the
tubes will be, seen from above, (as pointed out in the first figure)
when compared to each others. Compare the colors by looking through
the solutions from above with a white filter paper, illuminated
with good light, as the back ground. Like everyone expects the
intensity is the same in the two tubes. Now, predict what impact
on the intensity diluting one of the solutions, with water to
the double volume, will have. Most students predict that the solution
will be brighter, a few that it will be darker and very few that
the dilution will not affect the intensity of the color. The correct
prediction is ''No change'', this is obvious when considered that
the number of moles for the molecules that generates the color
is the same after dilution. When observed along the length of
the tube the observation passes the same number of coloring particles
no matter how large or small the volume is. The spontaneous prediction
is based on the experiences from observations made from the side
when a colored solution (for instance a fruit syrup)is being diluted.
As mentioned earlier in the text from this point the students
discuss how this result can be used for generating a formula and
a method for determining the mole concentration of the solution
they have prepared by using the reference solution with the known
concentration 0.10 M. They usually generate the equation from
which they can work out a method without any problems. To construct
a simple and effective method using their observations and their
equation takes them a little more of intellectual work. The most
common way to determine the concentration that the students work
out is to pour the 10 mL solution they have prepared into one
tube and then they fill the other tube with a Beral pipette until
they have the same intensity in color in the two tubes. They measure
the heights (h1 and h2) of the solutions and after using the values
to solve the equation they have a result that is within 10 % error
from the correct value. Finally they convert the instructed mass
concentration (15.0 g/L) into moles per liter and find that the
two results are comparable.
Summary:
Two experiments in sequence use the same salt for quantitative
measurements, one is using a gravimetric method and one is using
a colorimetric method. One is determining the number of crystal
water molecules in copper sulfate, one is determining the mole
concentration of the same salt in a solution that they have prepared
with high accuracy. The two experiments give use to a lot of the
theory in stoichiometrics that they have learnt during lectures
and in problem solving. The use of the same salt in both experiments
improves the value of the second experiment as this offers them
a situation where they can draw conclusions built on experiences
that they picked up in the first experiment. In order to get good
results they have practiced methods they can use in other occasions,
they have been trained to work with accuracy combined with awareness
of the benefit to do so. My experience is that the students are
engaged in these two experiments and they give feed back that
this sequence is effective in bringing understanding to their
studies.
 Top
Top

 CEJ Vol. 7, No. 2, Contents
CEJ Vol. 7, No. 2, Contents
 The later experiment contains a discovery experience
when the students find out that the color intensity (seen along
the length of the tube it is in, see the arrows in the tubes in
the figure below) is independent of diluting. The observed result
is surprising and it is challenging the students to understand
why. When the students are able to explain the phenomena as "same
color means same number of moles" (n1=n2)
then they are also able to convert the formula into c1V1=c2V2,
and because the tubes are identical the bottom areas are equal
which simplifies the formula to c1h1=c2h2
( n is number of moles, c is the mole concentration, V is the
volume and h is the distance through the liquid when the color
intensity was visually estimated). The instruction for preparing
the analyzed solution is given in g/L, the colorimetric result
is given in moles/L. When the grams per liter are converted into
moles/L the students see the relation between mass and moles.
Beside a lot of cognitive work they also gain a lot of manipulative
skills useful in most common laboratories.
The later experiment contains a discovery experience
when the students find out that the color intensity (seen along
the length of the tube it is in, see the arrows in the tubes in
the figure below) is independent of diluting. The observed result
is surprising and it is challenging the students to understand
why. When the students are able to explain the phenomena as "same
color means same number of moles" (n1=n2)
then they are also able to convert the formula into c1V1=c2V2,
and because the tubes are identical the bottom areas are equal
which simplifies the formula to c1h1=c2h2
( n is number of moles, c is the mole concentration, V is the
volume and h is the distance through the liquid when the color
intensity was visually estimated). The instruction for preparing
the analyzed solution is given in g/L, the colorimetric result
is given in moles/L. When the grams per liter are converted into
moles/L the students see the relation between mass and moles.
Beside a lot of cognitive work they also gain a lot of manipulative
skills useful in most common laboratories.

 Top
Top