Figure 1. Unique structures of 1,2,3-tribromobenzene (Br = 79Br and Br = 81Br).
Chemical Education Journal (CEJ), Vol. 7, No. 2 /Registration No. 7-20/Received September 17, 2003.
Department of Physical Sciences, Prince George's Community College, Largo, Maryland 20774, USA
E-mail: rgross@pgcc.edu
Abstract
Introduction
Results and Discussion
Conclusions
References
A sample of a compound that contains a few chlorine and bromine atoms in its molecular formula is composed of multiple sets of unit samples of molecules that replicate the molecular composition of the bulk sample. The number of molecules in a unit sample is stoichiometrically related to the number of bromine and chlorine atoms in the formula and contains unique molecules and duplicates of them or non-unique molecules. It is shown that the number of chlorine atoms in the formula is a function of the fraction of non-unique molecules in the unit sample. This relationship allows the number of chlorine atoms and hence the number of bromine atoms in the formula of an unknown compound to be calculated, because the molecular composition of a unit sample can be found from the molecular-ion peaks in the mass spectrum of the compound. Unit samples help instructors and students understand the nature of molecular compositions.
Chlorine exists as 35Cl and 37Cl in a 3.129 to 1.000 ratio, and bromine exists as 79Br and 81Br in a 1.028 to 1.000 ratio [1]. A sample of a compound of general formula CxH2x+2-m-nBrmCln contains a number of molecules that vary in mass because of these isotopes. Molecules of identical mass may differ in the location of the halogens in the structure. For example, the three structures shown in Figure 1 are all 1,2,3-tribromobenzene of integral mass 314, but they differ by the location of the 79Br and 81Br nuclides relative to the asterisk marker. The asterisk allows the structures to be treated as unique molecules for statistical accounting.
Figure 1. Unique structures of 1,2,3-tribromobenzene (Br
= 79Br and Br = 81Br).
The growth in the number of molecules by mass and isotopic structure as m and n increase can be determined when the following assumptions are made. Carbon and hydrogen in the molecules are assumed to be composed solely of 12C and 1H with no 13C, 2H, or 3H present, and the isotopes of bromine and chlorine are assumed to be present in exactly 1:1 and 3:1 ratios, respectively. Beynon [2] excluded 13C and 2H in his calculations but used the actual ratios of bromine and chlorine isotopes to calculate the mass-spectral peaks of compounds containing bromine and chlorine. He then matched the resulting spectral patterns to those of compounds containing unknown numbers of chlorine and bromine atoms to determine the actual values for n and m. The technique of finding n and m by pattern matching is still in use [3]. This paper shows how molecular compositions vary with m and n and how this variability allows values to be calculated for n and m without pattern matching. The variables in the following discussion are defined in Table 1.
Table 1. Definitions of variables
|
|
Definition |
|
|
The number of Br atoms in a formula |
|
|
The number of Cl atoms in a formula |
|
|
m + n |
|
|
The number of molecular-ion peaks in a mass spectrum attributable to the presence of chorine and bromine atoms |
|
|
A unit sample (i.e., the smallest representative sample of a bulk sample that contains only molecules containing Br and Cl atoms in a 1:1 and 3:1 ratio of isotopes, respectively, plus the lowest-mass nuclides of other elements) |
|
|
The number of statistically unique molecules in a unit sample (i.e., the number of molecules that would be present were both chlorine and bromine to exist in a 1:1 ratio of isotopes) |
|
|
N - u |
The growth in the number of molecules as the number of bromine atoms increases can be shown by starting with bromobenzene and then introducing a second and third bromine atom as shown in Figure 2.
Figure 2. Unit samples of bromobenzene, 1,2-dibromobenzene and
1,2,3-tribromobenzene with the masses shown in parenthesis.
As seen in Figure 2, N is the minimum number or the unit sample of molecules required to describe a bulk sample of molecules, and the value of N doubles with each additional Br atom. A bulk sample of molecules is made up of multiple sets of unit samples. Because bromine is assumed to exist in a 1:1 ratio of 79Br to 81Br, every molecule in each unit sample is unique with respect to the asterisk. When molecules of like mass are aggregated, the ratios of masses separated by two amu form the patterns 1:1, 1:2:1, and 1:3:3:1. These patterns are the same as the mass-spectral isotope patterns of the corresponding molecular ions. Excluding the hydrocarbon portions of the molecules, the same results for N as those shown in Figure 2 are obtained by expansion of the binomial of equation 1 [4] for each value of m. In equation 1, Br = 79Br and Br = 81Br.
|
|
(1) |
For m = 1, 2, and 3, respectively, N = 2, 4, and 8; (Br + Br)1 = Br + Br or 2; (Br + Br)2 = BrBr + BrBr + BrBr + BrBr or 4 and (Br + Br)3 = BrBrBr + BrBrBr + BrBrBr + BrBrBr + BrBrBr + BrBrBr + BrBrBr + BrBrBr or 8. When the linear formulas are aggregated by masses of the molecules they represent, the 1:1, 1:2:1 and 1:3:3:1 mass-spectral patterns emerge. Though BrBrBr and BrBrBr are structurally identical, they are distinguished statistically by the asterisks in Figure 1 or by the order in which the blue and red Br atoms are listed (i.e., BrBr differs statistically from BrBr by the order in which red and blue atoms appear). Thus, for bromine every molecule within a unit sample is distinguishable from all of the others.
The unit samples of molecules containing only chlorine atoms differ from those that contain only bromine, because chlorine exists in a 3:1 instead of 1:1 ratio of isotopes. A unit sample of four molecules is required for chlorobenzene and 16 molecules for 1,2-dichlorobenzene as shown in Figure 3.
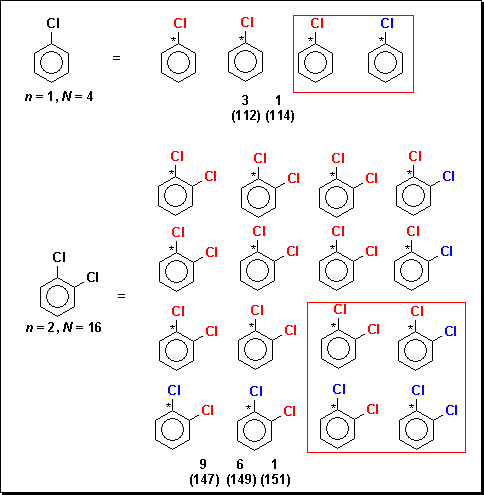
Figure 3. Unit samples of chlorobenzene and 1,2-dichlorobenzene
with the unique structures in red boxes and the masses shown in
parenthesis.
As seen in Figure 3, N quadruples when n increases by 1. The expansion of the binomial shown in equation 2, in which Cl = 35Cl and Cl = 37Cl, generates the unit samples shown in Figure 3.
|
|
(2) |
For n = 1, 2, and 3, respectively, N = 4, 16 and 64; (3Cl + Cl)1 = 3Cl + Cl or 4; (3Cl + Cl)2 = 9ClCl + 3ClCl + 3ClCl + 1ClCl or 16 and (3Cl + Cl)3 = 27ClClCl + 9ClClCl + 9ClClCl + 3ClClCl + 9ClClCl + 3ClClCl + 3ClClCl + 1ClClCl or 64. When molecules of like mass are aggregated, the 3:1, 9:6:1 and 27:27:9:1 mass-spectral isotope patterns emerge. It is seen from the expansions of equation 2 that the number of statistically unique molecules, u, is the number of linear formulas separated by plus signs or 2, 4, and 8 for n = 1, 2, and 3, respectively. The formulas enclosed by red boxes in Figure 3 represent the unique molecules in the unit samples of chlorobenzene and 1,2-dichlorobenzene. For chlorobenzene, N = 4, and u = 2. The difference N - u is the number of non-unique molecules q. The results for bromine and chlorine taken separately are summarized in Table 2.
Table 2. Values of N, u and q
as m and n increase separately
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The data in Table 2 show that all of the molecules in the bromine-containing compounds for which n = 0 are unique. Hence, q = 0 in each case. The value of u increases for chlorine exactly as it increases for bromine; therefore, u can be considered to represent the number of molecules that would be present in a unit sample if chlorine were present in a 1:1 ratio of isotopes like bromine instead of the 3:1 ratio. Likewise, q, the number of non-unique molecules in a unit sample, arises because of the "extra" two 35Cl nuclides in compounds containing chlorine. Because q is zero for all bromine-only compounds and rises rapidly with n for chlorine-containing compounds, the following question may be posed. Is it possible to determine the value of n in compounds that contain both bromine and chlorine by the growth in q? Figure 4 shows how N and u increase as both m and n increase.
Figure 4. Unit samples vs increasing values of m and n
with the number of molecules by increasing mass shown in parenthesis.
The unit samples shown in Figure 4 may be generated by expansion of the binomial pair of equation 3 (i.e., equation 3 is the unit-sample equation for compounds that contain bromine and chlorine).
|
|
(3) |
For example, for m = 1 and n = 2, N = 9BrClCl + 3BrClCl + 9BrClCl + 3BrClCl + 3BrClCl + BrClCl + 3BrClCl + BrClCl. In this example, u = 8, N = 32, and the pattern according to ascending masses two amu apart is 9:15:7:1. The growth of the molecules shown in Figure 4 is summarized and generalized in Table 3.
Table 3. Values of N, u and q
as m and n increase together
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The value of q is given by equation 4.
|
|
(4) |
The fraction of non-unique molecules in a unit sample, q/N, is given by equation 5.
|
|
(5) |
From Table 3, u = 2m2n and N = 2m4n. Substitution of these equalities for u and N on the right side of equation 5 gives equation 6, which is simplified to equation 7.
|
|
(6) |
|
|
(7) |
Equation 7 shows that the fraction q/N of non-unique molecules in compounds that contain chlorine and bromine is a function of n and independent of m. The data in Table 4 shows that the fraction q/N rises from 1/2 for n = 1 to 0.999 for n = 10 and approaches 1 as an absolute maximum.
Table 4. The fraction q/N in
a unit sample vs n and m
| q/N | 0 | 0 | 0 | 1/2 | 1/2 | 1/2 | 3/4 | 3/4 | 3/4 | 7/8 | 15/16 | 31/32 | ... | 0.999 |
| n | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 2 | 3 | 4 | 5 | ... | 10 |
| m | 1 | 2 | 3 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 0 | 0 | ... | 0 |
The fraction q/N changes only when n changes. Bromine atoms make no contribution to the value of q/N either when chlorine is absent or when chorine is present in the structure. When chlorine is absent, all of the molecules are unique. When chlorine is present, q and N both double with the addition of each bromine atom; therefore the ratio q/N remains constant. The values of q and N can be determined from a mass spectrum; therefore, the value of n can be calculated by equation 7. Consider the mass spectrum of 1-bromo-2,3-dichlorobenzene shown in Figure 5 [5].
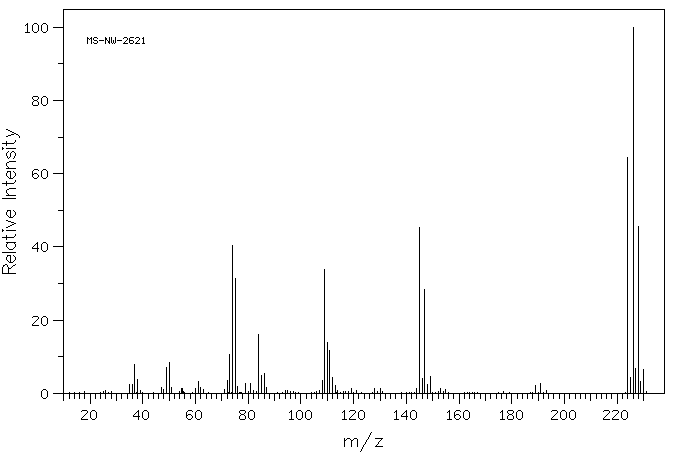
Figure 5. The mass spectrum of 1-bromo-2,3-dichlorobenzene reproduced
from reference 5
The molecular-ion cluster contains four peaks attributable to bromine and chlorine isotopes at m/z 224, 226, 228 and 230. The relative intensities of these peaks are shown in Table 5; the minor peaks at odd m/z values are omitted.
Table 5. Molecular-ion intensities for 1-bromo-2,3-dichlorobenzene
| Mass | Relative Intensity | Normalized Intensity |
| 224 | 64 | 11 |
| 226 | 100 | 17 |
| 228 | 45 | 8 |
| 230 | 6 | 1 |
The 11:17:8:1 set of normalized intensities corresponds to the 9:15:7:1 unit sample of molecules shown in Figure 4. The value of N for the number of molecules producing the real pattern is 37 as opposed to 32 for the calculated value. The number of peaks in the halogen-produced cluster is four. Because fluorine and iodine are monoisotopic, the number of chorine and bromine atoms in the formula causing these peaks is one less than the number of peaks [6]. If A is the sum of bromine and chlorine atoms and M is the number of molecular-ion peaks attributable to bromine and chlorine isotopes, then equations 8 and 9 show the known relationships among these variables.
|
|
(8) |
|
|
(9) |
From Table 5, M = 4; therefore, A = 3. Given the value of A, the value of u may be calculated, because u = 2m2n or 2A. For A = 3, u = 23 or 8. For 1-bromo-2,3-dichlorobenzene, N = 37, u = 8 and q = 29; therefore, q/N = 29/37 or 0.783. The value of n may now be calculated by substituting 0.783 for q/N into equation 7. The calculation is shown by equations 10-13.
|
|
(10) |
|
|
(11) |
|
|
(12) |
|
|
(13) |
The value of m follows from equation 8; m = 3 -
2 or 1. Thus, both n and m may be calculated from
the mass spectral data in Table 5. These calculations validate
the premise that the value of n might be found from the
fraction of non-unique molecules in a sample. That n must
be a whole number compensates for the 0.033 difference (i.e.,
0.783 - 0.750) between the value of q/N found for
1-bromo-2,3-dichlorobenzene and that calculated by equation 7.
The 0.033 difference is attributable to the assumptions underlying
the calculation.
Equation 7 is mathematically identical to equation 17, a simpler three-variable equation; the identity of the two equations may be shown as follows. Substituting N - u for q into equation 7 gives equation 14, and multiplying both sides of equation 14 by N gives equation 15. Rearranging terms results in equation 16.
|
|
(14) |
|
|
(15) |
|
|
(16) |
Equation 16 shows that the number of molecules in a unit sample is directly proportional to the number of unique molecules in the unit sample, and N = u when n = 0. That is, equation 16 gives the same results as shown by the structures in Figures 2-4. Because u = 2m2n and m + n = A, then u = 2(m + n) or 2A. Substituting 2A for u into equation 16 gives equation 17.
|
|
(17) |
Equation 17 is a unit-sample equation that has been derived directly from a variant of equation 3 [7]. The values of N and A may be found from the data in Table 5, leaving only n to be calculated (i.e., 2n = 37/8 = 4.6 or n = 2). The foregoing analysis shows that the theoretical basis for equation 17 is the fractionation of the molecular compositions of compounds that contain bromine and chlorine into predictable sets of unique and non-unique molecules. Because the number of unique molecules u is given by 2A, 2n is the factor by which the number of unique molecules must be multiplied to give the total number of molecules N in a unit sample.
Table 6 shows 14 additional examples for which equation 17 gives the correct values for n and m directly from actual mass spectral data. Some of the compounds also contain nitrogen and oxygen atoms.
Table 6. Compounds for which n and m have
been determined from mass-spectral data
| Isotope pattern (normalized) |
N | A | N /2A = 2n | n | m | Compound name |
| 10:6:1 | 17 | 2 |
|
2 | 0 | m-dichlorobenzene |
| 78:100:48:10:1 | 237 | 4 |
|
4 | 0 | 1,2,4,5-tetrachlorobenzene |
| 3:1 | 4 | 1 |
|
1 | 0 | chlorobenzene |
| 1:3:3:1 | 8 | 3 |
|
0 | 3 | 1,3,5-tribromobenzene |
| 3:8:5:1 | 17 | 3 |
|
1 | 2 | 2,6-dibromo-4-chloroaniline |
| 26:50:32:8:1 | 117 | 4 |
|
3 | 1 | 1-bromo-2,3,5-trichlorobenzene |
| 1:2:1 | 4 | 2 |
|
0 | 2 | 1,2-dibromo-4-nitrobenzene |
| 30:29:9:1 | 69 | 3 |
|
3 | 0 | 1,3,5-trichloro-2-nitrobenzene |
| 4:5:1 | 10 | 2 |
|
1 | 1 | m-bromobenzoyl chloride |
| 27:26:8:1 | 62 | 3 |
|
3 | 0 | 2',3',4'-trichloroacetophenone |
| 3:4:1 | 8 | 2 |
|
1 | 1 | 4-bromo-2-chloro-1-fluorobenzene |
| 10:17:7:1 | 35 | 3 |
|
2 | 1 | 2-bromo-1,3-dichlorobenzene |
| 3:4:1 | 8 | 2 |
|
1 | 1 | bromochloromethane |
| 3:8:5:1 | 17 | 3 |
|
1 | 2 | 2,6-dibromo-p-benzoquione-4-chloroimide |
The assumptions allow n and m to be found for compounds that are typically encountered in undergraduate chemistry courses (i.e., compounds for which the number of carbon atoms is less than 20 and the sum of bromine and chlorine atoms is less than 6). The number of non-unique molecules in a unit sample of molecules provides a theoretical basis for finding n in a compound of formula CxH2x+2-m-nBrmCln by either equation 7 or 17, which are equivalent. The presence of oxygen or nitrogen in the formula does not interfere with the analysis. The concept of unique molecules should be useful in chemistry courses where the growth of molecules as a consequence of isotopes is considered. This work was supported by the National Science Foundation of the United States by Grant DUE-0202431.
1. NIST web site: http://physics.nist.gov/PhysRefData/Compositions/index.html(accessed September 2003).
2.Beynon, J. H. Mass Spectrometry and Its Applications to Organic Chemistry, Elsevier: Amsterdam, 1960; p 413.
3. Smith, R. M., Busch, K. L. Understanding Mass Spectra- A Basic Approach, Wiley: New York, 1999; pp 52-53.
4. Smith, R. M., Busch, K. L. Understanding Mass Spectra- A Basic Approach, Wiley: New York, 1999; p 55.
5. SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/menu-e.html (accessed September 2003).
6. Lee, T. A. A Beginner's Guide to Mass Spectral Interpretation, Wiley: New York, 1998; p 55
7. Gross, Jr., R.A. Chem. Educator [Online] 2003 8, 182-186; DOI 10.1333/s00897030683a.