Promotion of Experimental Problem-solving
Skills based on Unknown Chemicals Exploration Experiment
Citation: Jhy-Ming Horng, Hsiao-Hsin Tsai
Department of Chemistry, National Taiwan Normal
University
Address: 88, Sec.4,
Ting-chou Road, Taipei 11650, Taiwan, R. O. C.
Keywords:
Inquiry-Based/ Discovery Method; Introductory/High School Chemistry; Laboratory
Instruction; Problem-based Learning; Qualitative Analysis.
Abstract
The qualitative analysis of unknown chemicals is adapted for talented
senior high school students in Taiwan. Thirty-two students, grouped in pairs,
were asked to design a procedure for the identification of sixteen unknowns.
The problem-solving process and strategies devised by the students can be
generally divided into three types: working backward, means-ends analysis, and
reasoning forward strategy. Successful and unsuccessful performances are
related to the procedural and domain-specific knowledge of students. The
results indicate that although the strategy of “working forward” is more effective,
an abundant and well-organized domain-specific knowledge is the key to success.
Furthermore, cross-examination is very important in the identification of
unknowns; many mistakes can be avoided by checking the prediction with the
results. When students attempt to construct a set of efficient, logical
thinking strategies, they probably encounter difficulties and become
frustrated, which may offer the opportunity to improve their problem-solving
skills. In addition, a problem-solving oriented experiment can stimulate
students to improve their understanding of chemical concepts and solve problems
through the use of procedural and conceptual knowledge. The unknown exploration
experiment is a well-designed unit that is intended to assess student performance
in terms of experimental problem solving and help them connect conceptual
understanding to problem solving in the real world.
Promotion of Experimental Problem-solving Skills based on Unknown Chemicals
Exploration Experiment
Jhy-Ming Horng, Hsiao-Hsin Tsai
Department of Chemistry, National Taiwan Normal
University
Address: 88,Sec.4,
Ting-chou Road, Taipei 11650, Taiwan, R.O.C.
The qualitative analysis of unknown chemicals is a useful exercise
that develops senior high school students’ experimental problem-solving skills.
Through inquiry-based experiments, students can pursue their interest in
chemistry, familiarize themselves with the scientific approach, and enhance
their higher-order thinking abilities, such as problem solving and logical reasoning.
In traditional teaching methods, laboratory manuals serve as “how to” recipes
for students. However, performing experiments step by step to achieve the
correct outcome often leads to the result that students conclude chemistry to
be unexciting (1). The major reason for this arises from the fact that
traditional teaching methods are only focused on obtaining so-called good
experimental results, while a true understanding of chemistry is often largely
ignored. In order to help students develop understanding of chemistry, we
provided students with opportunities to conduct inquiry-based experiments.
These learning experiences can help them to develop efficient and effective
problem-solving skills as well as strategies that generally lead to successful
solutions to real problems. To assist students in obtaining these skills and
strategies, it would be desirable to design effective instructional materials
in teaching (2).
The unit on the qualitative analysis of unknown chemicals is one of the major
topics in high school chemistry for grades 10 and 11 in Taiwan. An analysis
scheme for the identification of unknown white solids has been described in The Journal of Chemical Education (1-2).
The unknown chemicals exploration experiment can be adapted to different levels
of instruction. All procedures can be done with micro scale equipment. The case
shown here involves 32 grade 10, talented senior high school students, grouped
in pairs, who were asked to design a qualitative analysis process for sixteen
unknowns as an end-of-year project. In this paper, a problem-solving approach
for the identification of sixteen solids is proposed, and the strategies used
by the students are also discussed. Well-designed to assess students'
experimental abilities and help them connect conceptual understanding to real
problem solving, this exploration experiment has successfully served this
purpose. All 32 students involved consistently considered this experiment the
most challenging and their favorite.
The Expected Problem –Solving
Strategies and Skills Used by Students
A strategy defined
by Gagné (3) is a goal-directed sequence of mental operations. General
problem-solving strategies are activities that can improve the search for a
solution across a wide variety of problems. According to research on problem
solving, three expected strategies are “working backward”, “means-ends analysis” and “reasoning
forward”.
Working
backward
One way to limit the
search for a solution is to “work backward” from the desired goal. The key to
working backward is to decompose the initial goal into a set of subgoals that
imply the solution of the original goal. Reasoning backward involves setting
goals and subgoals and keeping track of them. The problem solver can then focus
on solving each of the subgoals independently (4). A powerful form of
working backward is referred to as means-ends analysis (3).
Means-ends analysis
The crucial step in means-ends analysis is selecting an operation that
reduces the functional difference between the current situation and the goal (3).
If one does not possess knowledge of such operations, one cannot use the
means-ends strategy. The success of means-ends analysis depends on the quality
of one's declarative knowledge. If students' declarative knowledge of functional
operations in the chemistry domain is deficient, they will have difficulty
performing means-ends analysis.
Working forward
Another way to limit a search is referred to as "working
forward", which involves performing whatever actions occur to one in response
to a given problem (3). Working forward is much simpler than
means-ends analysis. One examines the current situation and performs operations
to change it. The operations one selects are not constrained by the goal as
they are in means-ends analysis; therefore they may sometimes lead one in
fruitless directions. Working forward eliminates the need of keeping track of
subgoals. However, to successfully reason forward, one must know which of the
many possible forward inferences are relevant to the final solution.
Working forward functions when the operations suggested by the current
situation are the ones that lead to the goal. If the current situation suggests
misleading operations, working forward will not lead to the goal. Means-ends
analysis, therefore, is more powerful, because it selects only goal-relevant
operations.
The strategies used by expert and novice problem solvers differ (5).
Novices used the means-ends analysis. They worked backward from the subgoals.
Experts, in contrast, worked forward by well-organized domain-specific knowledge.
The strategy of the novice is called data-driven or search-driven, but the
expert's is schema-driven (6).
Problem-solving skills
Lyle and Robinson (7) suggested that
problem-solving skills include obvious elements such as the ability to read, to
perform experimental manipulations, to check results, to check that no
information is overlooked, and to check that the problem actually presented
was, in fact, solved. Other elements involve interpreting, representing, analyzing,
planning, execution, and evaluation.
To succeed in identifying all compounds in the unknown exploration
experiment, students must plan a set of efficient, logical thinking strategies.
In addition, students must examine their strategy repeatedly and adjust it as
needed. This represents a challenging task and requires the ability to keep
track of all the useful information as well as an adequate combination of
skills and strategies.
Context
The sample consists of 32 grade 10, mathematics
and science talented students in a senior high school. The school is a first-rate one in Taiwan.
All 32 students have taken introductory chemistry in the first semester and chemistry
topic research as elective in the second semester of the freshman year. We
perform this experiment as an end-of-year project without informing students
first in the course of chemistry topic research. Thirty-two students, grouped in pairs, were asked to design
a procedure for the identification of sixteen unknowns in three hours.
The Experiment
Apparatus and Materials
battery, copper wires,
lamp, litmus paper, aluminum plate, copper plate, zinc plate
Chemicals
Given: 0.01 M AgNO3(aq)
, 0.1 M HCl (aq) , 0.1 M HNO3(aq), 0.1 M H2SO4(aq)
, 0.1 M NaOH(aq) ,
Unknown: BaCl2, Ba(NO3)2, Ba(OH)2,
CaCO3, CaSO4, Flour, KI, NaCl, Na2CO3,
NaNO3, NaOH, Na2SO4,
Na2S2O3
, Pb(NO3)2, Sugar, ZnSO4
|
Students’ guide You
can use the materials and chemicals provided to identify the unknown
chemicals, No.1 to No.16. Present your approach and describe all the
reactions you have observed. Your report should indicate what reagents you
used, your observations and conclusions, and equations for the reactions. Hint: You can use the table of
solubility rules for ionic compounds in water. |
Results and Discussion
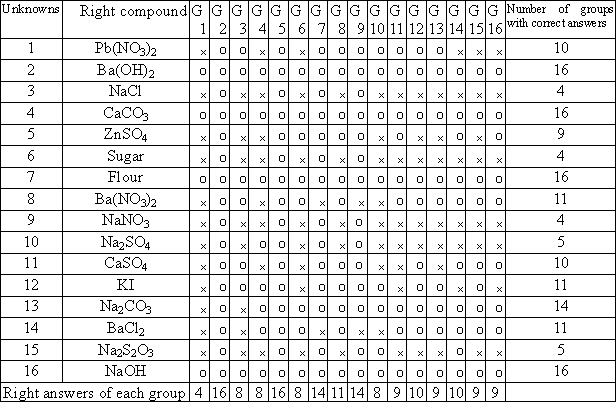
The result of experimental assessment is shown in Table 1. Table 1
shows that four groups, G2, G5, G7 and G9, can identify over 14 unknown
chemicals. We classify them into high-level problem-solving ability
cluster. Eleven groups, G3, G4, G6,
G8, G9, G110, G12, G13, G14, G15 and G16, can identify 8 to 13 unknowns. We
classify them into medium-level cluster. As for G1, we classify them into
low-level cluster because they can only identify four unknowns.
Table 1. The experimental assessment of 16 groups
To identify the sixteen unknowns, students must establish a set of
problem-solving strategies based on chemical concepts and logical reasoning.
Analyzing the reports and interview protocols of students, the problem-solving
procedures can be generally divided into three types: “working backward”,
“means-ends analysis”, and “reasoning forward”. We categorize the 16 groups
into suitable types according to their characteristics. We discover
an interesting phenomenon, that high-level cluster adapts type III-reasoning
forward, and that medium-level cluster adapts type II- means-ends analysis. The
strategy used by the low-level cluster is working backward. Below, we discuss each type with a representative group.
Type I-“working backward”
strategy
From our viewpoint, the strategy of the
lower-level cluster is classified into type I. The only group categorized in
this cluster is G1. The process used by G1 is shown without change as follows.
1. Observe the appearances of unknowns.
2. Test the unknowns' room-temperature solubility in water.
3. Use litmus paper to determine whether the soluble unknowns are
acidic or basic.
4. Add the given solutions, AgNO3(aq), HCl(aq),
H2SO4(aq), NaOH(aq) individually to all the
unknowns.
5. The result is listed in Table 2.
Table 2. The original
record of G1
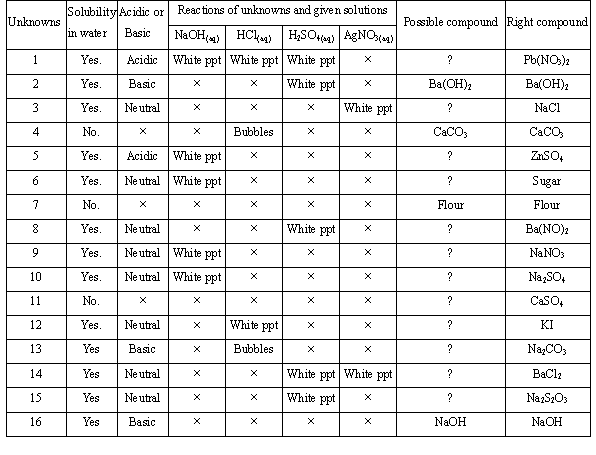
Note: The symbol “x”
means no visible reaction or unfinished test.
Comments:
Working backward aims at decomposing the
initial goal into several goals. G1 divides the problem, identifying 16
unknowns, into three goals, which are (1) testing the solubility in water of
unknowns, (2) testing the pH range of unknowns and (3) testing the reactivity
of unknowns with specific reagents. Then G1 divides the third goal into four
subgoals, which are testing the reactions of unknowns with given reagents: AgNO3(aq), HCl(aq),
H2SO4(aq), NaOH(aq .
The method used by G1 is similar to the
“search-driven” or “data-driven” strategy, which works backward from the
goal. Students generate a pathway to the solution and test to see whether it
can work. This method has two major drawbacks as an approach to the problem.
First, it does not provide criteria for selecting appropriate solutions to be
tested. Selecting reasonable solutions is critical so as not to waste time in
testing trials that work without achieving a positive result. Second, this
method involves generating every possible trial before testing it to see
whether it works. This process not only wastes time but can also be inconclusive.
In this case,
students have no idea how to perform the experiment. They simply mix chemicals
and decide what to do next, based on the results. In order to identify 16
unknowns by this way, one must operate 96 trials to complete Table 2 and
explain the results correctly. G1 tries to identify the 16 unknowns by analyzing
the 96 testing results in Table 2. However, Table 2 is too complicated to
analyze such that students cannot conclude the
following carefully. This puts a severe strain on the working memory and can
lead to errors. Thus, they cannot set subgoals anymore. In addition, there are
some mistakes in Table 2, as the result, perhaps, of the careless mixing of
unknowns or recording errors.
The reason why G1 failed is that they cannot set further subgoals
according to the differences between the current states and the goal. Besides,
owing to a lack of abundant chemical domain-specific and procedural knowledge,
they failed to identify the physical and chemical properties of the unknowns
and neglected the importance of cross-examination. Their knowledge base is
inadequate and incomplete; consequently, they are unable to plan a systematic
solving process and explain the experimental results in a meaningful way.
Type II - “means-ends
analysis” strategy
G3, G4, G6, G8, G10, G11, G13, G14, G15 and G16 are classified into this type. We
take G10’s problem-solving process as an example to discuss. The solving
process of G10 is described as follows.
1. Identify the compound that is moistened in the air as NaOH, based
on the appearance of the unknowns.
2. Test the unknowns' room-temperature solubility in water to
identify which sample is flour.
3.
Add 0.1 M H2SO4(aq)
to each of the other 14 unknowns to identify Ba2+ compounds (BaCl2,
Ba(NO3)2, Ba(OH)2) if white precipitate formed.
4. Add 0.1 M HCl(aq) to each of the other 11 unknowns to
check whether any gas is formed, then we can identify CaCO3 and Na2CO3.
The two compounds differ in their solubility in water.
5. Add 0.01 M AgNO3(aq) to each of the other 9 unknowns to
identify KI by the yellow precipitate.
6. Add the found KI(aq) to each of the other 8 unknowns to
identify Pb(NO3)2 by the yellow precipitate.
Treat the other 7 unknowns as described in the following flow
chart.
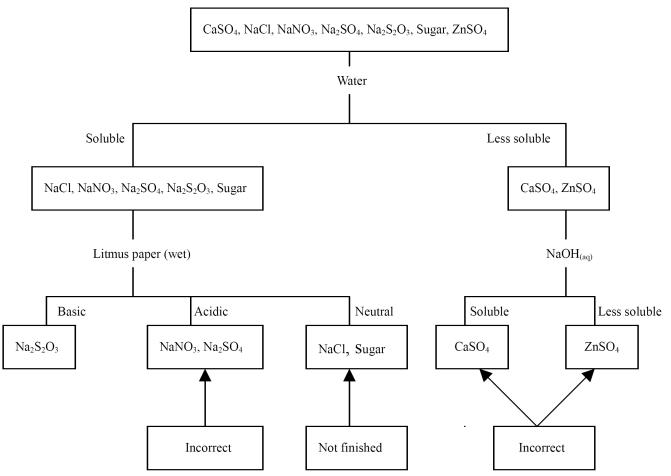
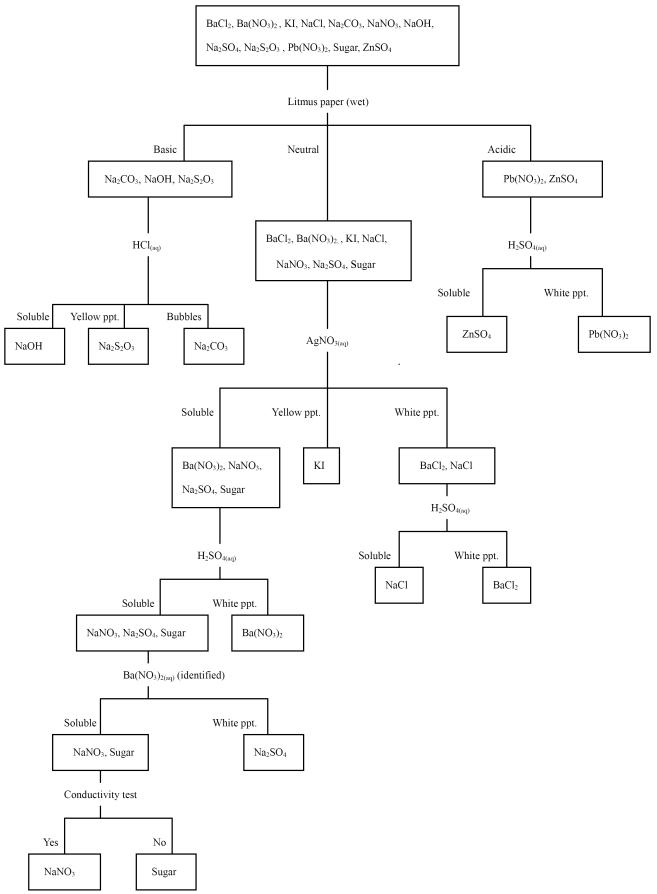
Figure 1. The qualitative analysis
scheme of G10
Comments:
The strategy of G10 is similar to a
“means-ends analysis”. Means-end analysis is a heuristic strategy for finding
subgoals. The process of analysis consists of two steps that are applied
repeatedly: (1) identifying the differences between the current state and the
desired goal, and (2) applying an operation to reduce one of these differences.
The strategy of G10 is to reduce the numbers of the unknowns gradually. Their
first goal is to find out NaOH, by means of observing the appearance of 16
unknowns. Next, they try to find out flour by observing the solutions of the
other 15 unknowns. Finally, they try to find the unknowns with Ba2+
by mixing the other 14 unknowns with H2SO4(aq). They make
use of all given reagents one by one to react with unknowns. Each operation can
identify some unknowns and reduce the number of unknowns little by little.
In addition to the “means-ends” strategy, G10 attempts to reason with chemical
concepts. Step 1 to 6 in G10’s solving process indicates that the chemical
reasoning is logical and correct. However, the three Ba2+ compounds
were not distinguished in step 3. Additionally, mistakes made in the final step
reveal that G10 did not make use of their knowledge relative to the chemical as
well as the physical properties of the unknowns, and failed to judge the pH and
solubility of chemicals. The result indicates that although the strategy of
“means-end analysis” is useful, abundant and well-organized domain-specific
knowledge is the key to success. Besides, the cross-examination is very
important in the identification of unknowns; many mistakes can be avoided by
checking the prediction from the results.
Type III- “reasoning forward” strategy
G2, G5, G7and G9 are classified into this type. We take G2’s
problem-solving process as an example to discuss. The solving process of G2 is
summarized as follows.
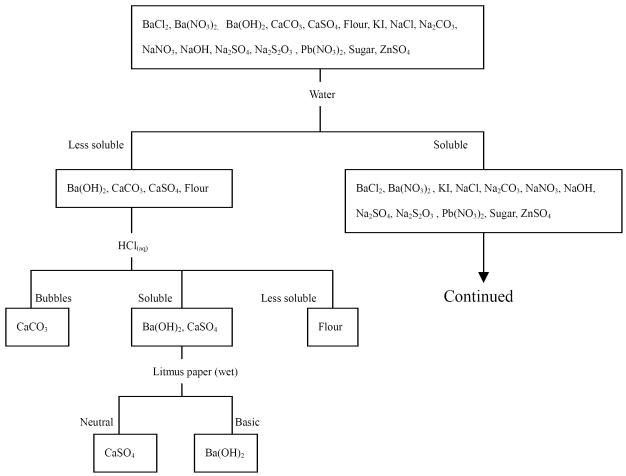
1. The process for identification is shown in the form of a flow chart in Figure 2.
2. We can
experimentally determine whether a substance forms an electrolyte solution by
testing the ability of the solution to conduct an electrical current. A DC
circuit device using the battery, copper wires, copper plates as electrodes,
and a lamp, can be set up to test whether or not ions are present in the
unknown solutions, provided that the solutions are not too dilute. If ions are
present, the solution completes the electrical circuit, and the lamp glows.
Comments:
Analyzing the solving strategy of G2, undoubtedly,
they displayed an effective and efficient solving process. The unknowns are
initially divided into two groups based on their solubility in water. Among the
16 solids, 12 are water-soluble and four are less soluble. The criteria of
categorization include solubility, a pH test, electrolyte/non-electrolyte and
reactions with specific reagents. This represents a successful case in
“reasoning forward” strategy. The students had a greater grasp of the concepts
and were able to systematically organize their chemical knowledge. Therefore,
G2 can focus on how to solve and construct a logical as well as an efficient
plan of problem solving instead of operating by trial and error. In addition,
they continue to search for useful resources and make use of the knowledge
gained from the experimental process. This problem-solving approach is similar
to the expert's performance.
The novice solution typifies the method
of working backward, similar to the performance of G1. Novices start out by
working backward and slowly develop strategies that make forward inferences.
Experts and novices typically apply chemical principles in precisely the
opposite order. The differences between G1 and G2 are identical to those between experts and novices. There are also changes at the
strategic level, which is concerned with how students organize their solution
to an overall problem. The procedure of learning how to organize one's problem
solving is referred to as strategic learning.
Conclusions and Recommendations
Considering the above discussion and comments on three types of strategies, the results indicate that successful
and unsuccessful performances are related to the procedural and domain-specific
knowledge of students. Although the strategy of “working forward” is more
effective, an abundant and well-organized domain-specific knowledge is the key
to success. Furthermore, cross-examination is very important in the
identification of unknowns; many mistakes can be avoided by checking the
prediction with the results.
We suggest that chemistry teachers should start with 5 or 6
unknowns then raise difficulty by adding additional unknowns and choose the
harmless ones in our daily life or those used quite often in laboratory. The identification process could
vary from simple to complex as the number of unknowns’ increases. The collection
of unknowns should exhibit the comparative meaning of chemical properties, for example, compounds with the
same cations or anions.
A typical concern of educators is whether what
is taught in the classroom can be applied in the real world. The inquiry experiment
of unknown chemicals is a well-designed unit used to assist an
instructor in assessing
student performance in terms of experimental problem solving. When students
attempt to construct their scheme of analysis, they will likely encounter
difficulties and frustrations, which may offer opportunities to demonstrate
needed skills and abilities through the use of chemical concepts. The
problem-solving oriented experiment can motivate students’ thinking and help
them learn how to construct their framework of chemical concepts and to solve
the real problems in laboratory by using procedural and conceptual knowledge.
In this way, students can develop effective problem-solving methods and will
have more confidence in their ability to solve problems.
 Top
Top