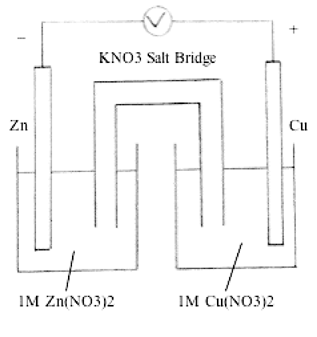
Figure 1. Galvanic Cell
E-mail:david@fau.edu
ABSTRACT
INTRODUCTION
ALTERNATE FORMS OF SALT BRIDGES
FINAL THOUGHTS
ACKNOWLEDGEMENTS
REFERENCES
Alternate approaches to building salt bridges for electrochemical cells are reviewed. Materials such as filter paper, cotton, semi-micro tubes, human beings, Soil Moist, vials, etc. have been used to construct salt bridges in instructional laboratory settings. Of these, a human salt bridge involving 1500 people (Silverman and Bunn, 1992) is an interesting example in terms of bringing the concept of salt bridges to life and making laboratory chemistry meaningful.
Alternate approaches to constructing salt bridges in galvanic cells for laboratory instruction are discussed. Common complaints about subjects such as chemistry are "boring", "hard" and "dry". Activities involving the construction and use of alternate forms of salt bridges in electrochemistry should provide opportunities for students to visualize the connection between classroom chemistry and real life materials. In an instructional laboratory setting it may be to the advantage of the learners to use a salt bridge that is easy to construct, consists of common materials and excites student interest in learning electrochemistry. Galvanic cells are electrochemical devices that convert chemical energy into electrical energy based on oxidation-reduction or redox reactions. The number of electrons lost and gained in the half-reactions must be equal in order to achieve a balanced redox reaction. In galvanic cells, the half-reactions take place in two separate electrode compartments. Electrodes are connected externally by an electrical conductor, and the compartments are connected through a salt bridge consisting of conducting electrolytes. A half-cell is one compartment with an oxidizing or reducing reaction, and a salt bridge is an electrolyte connecting two half-cells (Figure 1). A traditional salt bridge would consist of a U-shaped glass tube filled with an electrolyte solution such as KNO3(aq) in agar-agar (a colorless and odorless polysaccharide complex) whose ions will not react with the electrolyte or the electrodes.

For example, the cell reaction Zn(s) + Cu2+(aq) = Zn2+(aq) + Cu(s), where the oxidation of Zn produces Zn2+ ions into the anode half-cell. This positive charge must be neutralized to facilitate further oxidation. Likewise, the reduction of Cu2+ produces an excess negative charge in the cathode half-cell. Salt bridges allow the migration of these ionic charges from one half-cell to the other to maintain electroneutrality (Kennedy, 1984). In effect, a salt bridge minimizes the liquid junction potential, with the potential difference arising at the interface of electrodes and electrolytes. To maintain the cells as electrically neutral, the electrons released must travel through the wire resulting in electrical energy.
ALTERNATE FORMS OF SALT BRIDGES
A survey of the Chemical Abstracts and a subsequent review led to the following alternate forms of salt bridges used in instructional laboratory settings: filter paper (Joesten 1991; Scharlin, Battino and Boschman, 1991), cotton swab (Dobrzynski, 1996), semi-micro tube (McCullough, 1973), human beings (Brower, 1974; Scharlin and Battino, 1990; Silverman and Bunn, 1992), Soil Moist (Brooks and Brooks, 1994), and vial (Craig, Ackerman, and Renfrow, 1989). These salt bridges were tested by inservice teachers enrolled in a science education course. Further discussion will address the characteristics of these and other salt bridges for instructional laboratory experiments.
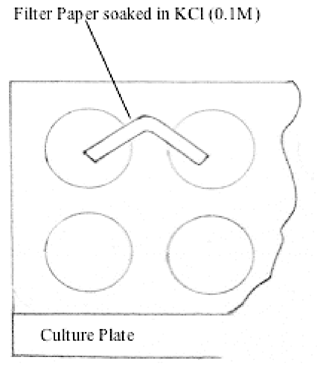
Filter Paper
Filter paper strips (1.5 cm x 2mm) soaked in 0.1 M KCl solution
are used to bridge various pairs of half-cells in a 24-well culture
plate (Joesten 1991). See Figure
2. Use a pair of tweezers to handle the KCl soaked filter
papers, and place them between two half-cell wells. Make sure
the filter paper strips are immersed in the liquids in adjacent
wells. Thick blotting paper or a set of four to five filter papers
soaked in an electrolyte also have been reported in the literature
as a salt bridge in a voltaic pile involving Cu/Zn and Cu/Pb metal
pairs (Scharlin, Battino and Boschman,
1991). Paper towel strips could be used in place of a filter.
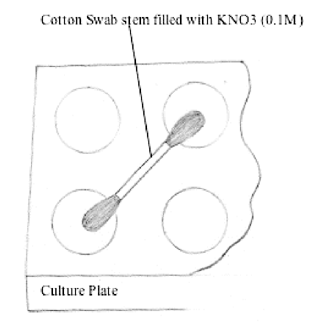
Cotton Swab
Using a syringe, inject 0.1 M KNO3 solution prepared
in 1.0 gram agar-agar into the hollow plastic cotton swab stem
(approx. 75 mm in length) (Dobrzynski,
1996) (Figure 3). (Note: Stems
of certain types of cotton swabs (e.g., Q-tips) are not hollow.)
Make sure the cotton tips on both ends of the swab are saturated
with the KNO3 solution. Use this cotton swab to bridge
various pairs of half-cells in a standard 12-depression spot plate.
Reported voltage measurement is within 0.03 V of the expected
values (Dobrzynski, 1996). Note:
To avoid any personal injuries, firmly hold the cotton swab stem
with a pair of tongs when injecting the KNO3 solution.
Semi-micro Tube
Capillary U-tubes diameter 0.04 - 0.05 mm and 10 - 1.5 cm in length
are suitable for making salt bridges and can be filled with the
bridge solution by capillary action (McCullough,
1973). The capillary tubes are made from 5 mm soft glass tubes
by appropriately heating and pulling them to reduce the
diameter. These semi-micro salt bridges lead to very little contamination,
and cause no observable voltage fluctuation.
Human Salt Bridge
There are several types of human salt bridges reported (Brower,
1974; Scharlin and Battino, 1990;
Silverman and Bunn, 1992). The "Longest
Human salt bridge" involved 1500 people holding hands in
series (Silverman and Bunn, 1992).
The last person on the left hand side of the bridge dips his/her
right index finger in one half-cell (Zn2+) and the
last person on the right side dips his/her left index finger in
the other half-cell (Cu2+). The recommended resistance
of the lowest setting of the voltmeter is 1010 Ohms,
considering the resistance of 1.5 x 107 Ohms due to
the large number of people holding hands.
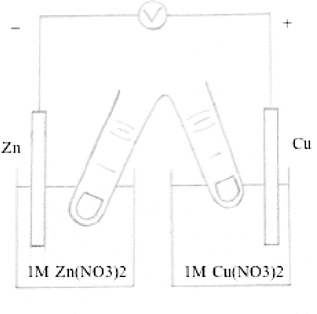
The simplest human salt bridge can
be constructed using the middle and index fingers of one hand
dipped in two half-cells (Brower, 1974).
See Figure 4. (Note: After the demonstration,
remember to wash fingers with running water.) This simple human
salt bridge produces identical voltage to that generated by a
traditional U-tube salt bridge containing NH4NO3
solution.
In another type of human salt bridge, ten pairs of electrodes (1/16 x 1/4 x 2 inch) are mounted on the left and right hand edges of a Plexiglas sheet (Scharlin and Battino, 1990). The electrode metals reported are Cu, Ag, Pb, Cd, Mg, Zn, Al, C, and Ni. See Figure5.
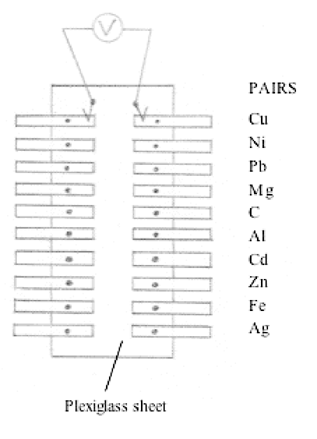
Using a pair of banana plugs, connect any two pairs of electrodes to a voltmeter. Press the left thumb against the electrode connected to the voltmeter on the left hand, and press right thumb against the electrode connected to the voltmeter on the right hand side, and note the voltage. (Contact-resistance and moisture on the fingertips may cause minor voltage fluctuations.) After multiple measurements, a standard deviation of 0.02 V to 0.04 V is reported (Scharlin and Battino, 1990). This human salt bridge is recommended for overhead demonstration when connected to an appropriate voltmeter suitable for overhead display. A simpler version of this demonstration involves a person being instructed to hold a zinc electrode in one hand, a copper electrode in the other hand, and connect the electrodes to a voltmeter.
Soil Moist
Soil Moist is 25% hydroxyethyl homopolymer, a dehydrated polymer
(synthesized by JRM Chemical Division, 13900 Broadway Avenue,
Cleveland, Ohio 44125) (Brooks and Brooks,
1994). Soil Moist swells in water. Fill the stem of a plastic
transfer pipette up to 3 cm with Soil Moist and then place it
in 0.5 M Na2SO4 solution for 3 minutes.
Cut off the bulb. Bend the stem containing the Soil Moist and
Na2SO4 to bridge electrolyte solutions in
a well-plate.
Vial
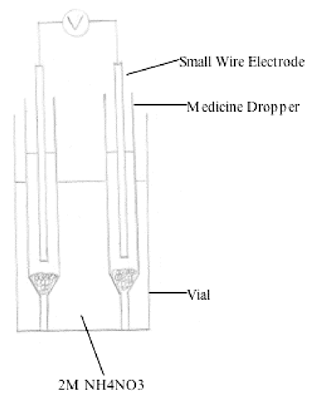
A 2-dram vial (17 mm x 16 mm) containing
2M NH4NO3 makes a very practical salt bridge
(Craig, Ackerman, and Renfrow, 1989).
Two stems of medicine droppers containing two half-cells, small
wire electrodes and cotton plugs are placed inside the vial salt
bridge (Figure 6). The observed voltage
was within 0.02 V and 0.1 V of the expected voltage. Trapped air
bubbles in the cotton plugs cause voltage fluctuation (Craig,
Ackerman, and Renfrow, 1989). Replacing the cotton plugs with
cellulose strips made from 12-14,000 molecular weight dialysis
tubing reduces air bubble trapping (Eggleton,
Williamson, and Johnson, 1990).
FINAL THOUGHTS
The half-cells linked by the salt bridges discussed have used
electrolytes such as Cu2+, Pb2+, Mg2+,
Zn2+, Fe2+, Fe3+, Ag+,
Sn2+, etc. Students should be encouraged to experiment
with other appropriate and safe materials for constructing salt
bridges. (e.g., A piece of unused lamp wick soaked in 0.1 M KCl
solution, flexible drinking straw filled with 0.1M KNO3
in agar-agar). A bendable drinking straw with accordion joint
bent into U-shape and the long side cut to the length of the short
side would be suitable for making a salt bridge (Figure
7).
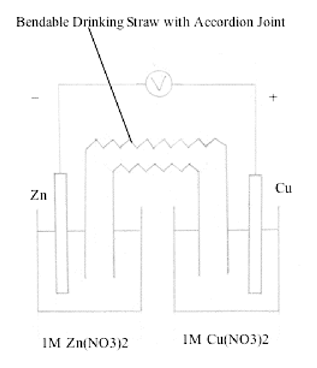
Fill the bridge with the conducting electrolyte before bending
into U-shape. This bridge is easy to construct and inexpensive.
Laboratory assignments involving galvanic cells might include
comparing the salt bridges for voltage output, voltage dissipation,
resistance, etc.
The cotton swab salt bridge is perhaps the most difficult one
to make since it involves injecting the electrolyte into the stem
of the swab. Teacher supervision is required. In some brands of
swabs, the stem is no longer hollow. The human salt bridge is
perhaps the easiest and most engaging bridge to construct. Inservice
teachers enrolled in a science education class who assisted in
verifying the alternate forms of salt bridges were unusually curious
and raised many interesting questions about galvanic cells and
electrochemistry which led to meaningful discussions. Approaches
to chemistry instruction should encourage student participation
in chemistry laboratories and make learning chemistry an enjoyable
experience.
ACKNOWLEDGEMENTS
The author would like to thank Karen Tobias, Amy Bingham, Lisa
Mikes, and Anne Marie Mayer for testing the suitability of the
salt bridges for classroom use, and Robyn Trainer for editorial
assistance.
Brooks, D. W., and Brooks, H. B. (1994). Salt bridge using soil moist. Journal of Chemical Education, 71(3), A73.
Brower, H. (1974). A human salt bridge. Journal of Chemical Education, 51(11), 744.
Craig, N. C., Ackermann, M. N., and Renfrow, W. B. (1989). Miniware for galvanic cell experiments. Journal of Chemical Education, 66(1), 85-86.
Dobrzynski, E. D. (1996). Voltaic cell measurements on a spot plate with a cotton swab salt bridge. Journal of Chemical Education, 73(1), A6.
Eggleton, G. L., Williamson, J. J., and Johnson, D. K. (1990). Membrane material for a Galvanic cell. Journal of Chemical Education, 67(6), 527.
Joesten, M. D. (1991). Experiments for chemistry 102B, Vanderbilt University, Nashville, TN.
Kennedy, J. H. (1984). Analytical chemistry principles. San Diego: Harcourt Brace Jovanovich, Inc.
McCullough, T. (1973). A semimicro salt bridge. Journal of Chemical Education, 50(11), 781.
Scharlin, P., Battino, R., and Boschman, E. (1991). The voltaic pile: A stimulating general chemistry experiment. Journal of Chemical Education, 68(8), 665.
Scharlin, P., and Battino, R. (1990). The human salt bridge. Journal of Chemical Education, 67(2), 156-157.
Silverman, L. P., and Bunn, B. B. (1992). The world's longest human salt bridge. Journal of Chemical Education, 69(4), 309-310.
 Top
Top
