Monosaccharide Cycles: A Method to Determine the General Stereochemical Relationships of both D and L Monosaccharides.
Mo Hunsen
Department of Chemistry, Kenyon College, Gambier, OH 43022
Phone 740-427-5091; Fax 740-427-5731
e-mail: hunsenm@kenyon.edu
Abstract . Using a ‘cyclic’ representation of both D and L monosaccharides, named monosaccharide cycles, a method for determining the stereochemical relationships of all (both D and L) monosaccharides is proposed. It would be a very useful tool for teaching Organic, Bioorganic, and Carbohydrate Chemistry and Biochemistry.
Keywords : Organic Chemistry, Biochemistry, Carbohydrates, Nomenclature, Stereochemistry.
Carbohydrates, proteins, and nucleic acids constitute the three most important biopolymers in living systems. Natural amino acids, the building units of proteins, differ only by their side chains and almost all of them have the L-configuration. Nucleic acids are phosphodiester-linked riboses or deoxyriboses to which one of four types of bases are attached. In contrast, the monosaccharides, the building units of carbohydrates, have multiple stereocenters. The presence of these multiple stereocenters contribute to the rich structural diversity of carbohydrates, enabling them to serve as ‘molecular (cellular) barcodes’. For example, studies have demonstrated that oligosaccharides are involved in a number of recognition events such as cell adhesion, metastasis, fertilization and embryonic development, amongst others.[1,2] In organic and carbohydrate chemistry, the monosaccharides are typically represented in a chart form [3-5] and their naming follows the IUPAC-IUBMB nomenclature.[6] Understanding the nomenclature and the stereochemical relationships between different monosaccharides can be diffcult for students. One of the important topics suggested to be covered in undergraduate education by BIO2010 is carbohydrate chemistry.[7] With the rise of a new era of glycomics, carbohydrates are increasingly being important especially in the pharmaceutical industries. One of the major obstacles in teaching carbohydrate chemistry is getting a handle on their name/structure and stereochemical relationships. The method proposed in this manuscript is expected to alleviate this obstacle. Reported herein is a simple method to determine the stereochemical relationships of D and L monosaccharides. This is based on a ‘cyclic’ relationship of the different monosaccharides and is termed the Monosaccharide Cycle or MoCycle for short. Instructions on how to draw the monosaccharide cycles is given in the supplemental information and discussions on how to determine the stereochemistry monosaccharides, based on the cyclic relationship between the different monosaccharides, are given below. The monosaccharide cycles (Figures 1-2), the generalized monosaccharide cycles (Figures 3-5), and the rules could be used as an end product, for example by printing and distributing the diagrams to students or the more curious and advanced students can practice drawing the diagrams by themselves. Not to imply learning by color, the connections (lines) in the diagrams are drawn as different type of lines and could be printed in black and white if need be. The rules for properly using the MoCycles i.e. what we get out of the MoCycles are given below.

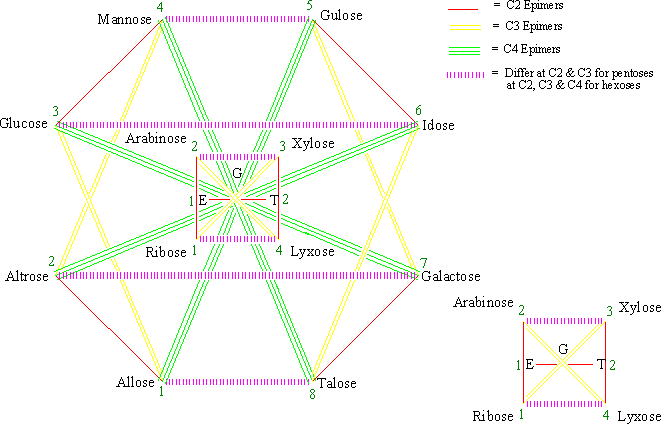
Figure 1 . The monosaccharide cycle of aldoses (G = glyceraldehyde, E = erythrose, and T = threose).
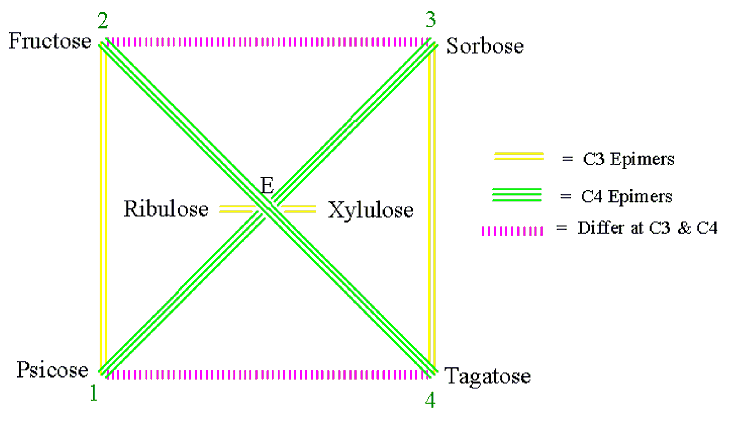
Figure 2 . The monosaccharide cycle of ketoses (E = erythrulose).
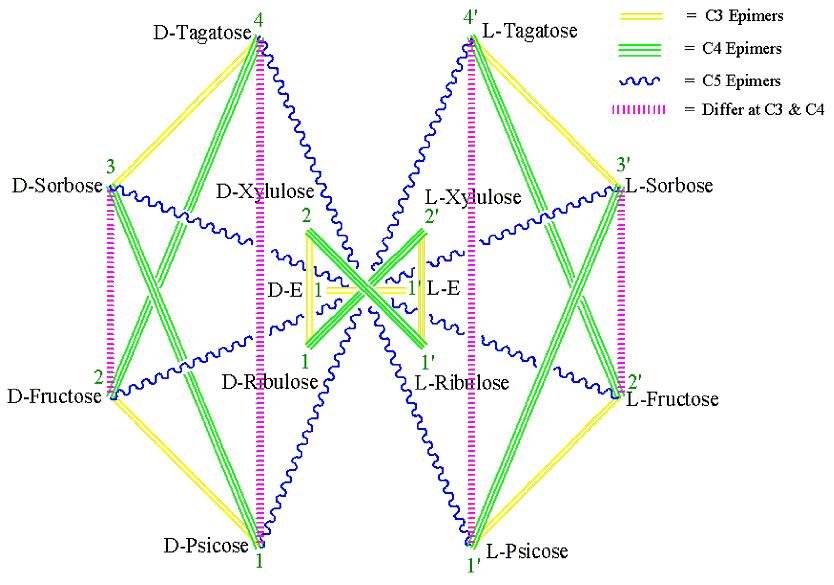
Figure 3 . Generalized MoCycle of Ketoses (E = Erythrose).
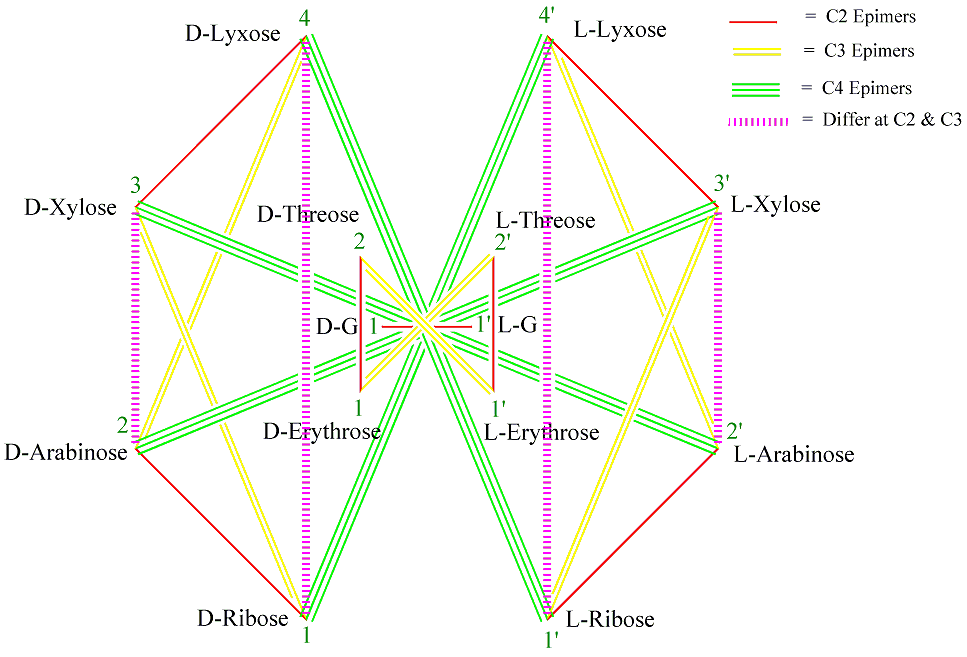
Figure 4 . Generalized MoCycle of Aldoses of five carbons or less (G = Glyceraldehyde).
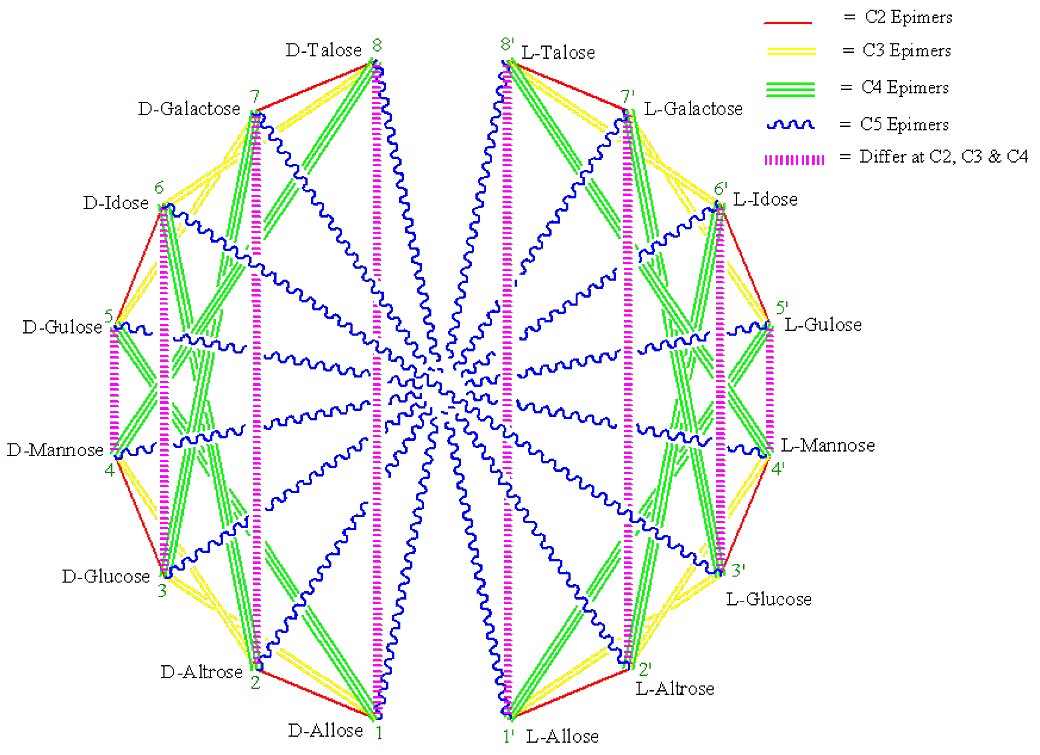
Figure 5 . Generalized MoCycle of Aldohexoses.
1. Monosaccharides connected by a single-red, double-yellow, triple-green and wiggly-blue (RYGB) lines (Figures 1-5) are C2, C3, C4 and C5 epimers, respectively.
2. Monosaccharides connected by a dashed-magenta line (Figures 1-5) have different stereochemistry at all carbons with the exception of the carbon next to the hydroxymethyl group, CX, which has the same configuration for all D sugars (or for all L sugars). For example, D-Ribose and D-Lyxose, and D-Arabinose and D-Xylose differ both at C2 and C3; D-Allose and D-Talose, D-Altrose and D-Galactose, D-Glucose and D-Idose, and D-Mannose and D-Gulose differ at C2, C3 and C4. And the same holds true for the L-monosaccharide pairs.
3. When two monosaccharides are connected by a dashed-magenta line (Figures 1-5), the D form of one and the L form of the other are CX-epimers. CX is C4 for aldopentoses and C5 for aldo- and keto-hexose. See the D-L monosaccharide pairs in Table 1 for examples.
4. For the same pair of monosaccharides (Figures3-5), the relationship between their D-D pair is the same as that of their L-L pairs, and that between D-L pair is the same as their L-D pair. For example, in Figures 4 D-Ribose and L-Lyxose are C4 epimers and so are L-Ribose and D-Lyxose.
5. Two monosaccharides differ by as many stereocenters as the fewest number of lines it takes to get from one to the other (Figures 1-5). For example, in Figures 1 D-Allose and D-Altrose connected by a single-red line differ at C2. Similarly, D-Allose and D-Mannose connected by a single-red and a double-yellow lines (two ways: by way of D-Altrose or by way of D-Glucose) differ at C2 and at C3.
6. As we go outwards from inside of the cycle (Figures 1-5), the monosaccharides add one CHOH group [i.e. two C2 epimers (C3 for ketoses) are formed]. For example, in Figures 1 Allose and Altrose from Ribose; Ribose and Arabinose from Erythrose, and Erythrose and Threose from Glyceraldehyde.
7. D-Monosaccharides on corner 1 (i.e. Glyceraldehyde, Erythrose, Ribose, Allose, Erythrulose, Ribulose, and Psicose) have all their hydroxyl groups on the right side of the Fischer Projection (Figure 6 and 7).[3] Hence they have the R-configuration for at all their stereocenters on their Fischer projection. Note that stereocenters on the Fischer projection with OH on the Right side has the R configuration and those with OH on the left side have the S configuration (Figure 6 and 7).[3] The opposite is true for L-monosaccharides.
8. The b-pyranoses of monosaccharides on corner 3 (Xylose, Glucose and the aldoketose sorbose) have all their hydroxyl groups at the equatorial positions. Their a -form will have only its C1 hydroxyl at the axial position (Figure 8).[3] Using these three monosaccharides as reference and with the help of the simple and the generalized MoCycles one can easily determine whether a given hydroxyl group is axial or equatorial for the other monosaccharides.
Table 1. Epimeric Monosaccharide Pairs.
C2 Epimers |
C4 Epimers
|
D-Erythrose and D-Threose D-Ribose and D-Arabinose D-Xylose and D-Lyxose D-Allose and D-Altrose D-Glucose and D-Mannose D-Gulose and D-Idose D-Galactose and D-Talose |
D-Ribulose and L-Xylulose D-Ribose and L-Lyxose D-Arabinose and L-Xylose D-Psicose and D-Sorbose D-Fructose and D-Tagatose D-Allose and D-Gulose D-Altrose and D-Idose D-Glucose and D-Galactose D-Mannose and D-Talose |
C3 Epimers |
C5 Epimers
|
D-Erythrose and L-Threose D-Ribose and D-Xylose D-Arabinose and D-Lyxose D-Psicose and D-Fructose D-Sorbose and D-Tagatose D-Allose and D-Glucose D-Altrose and D-Mannose D-Gulose and D-Galactose D-Idose and D-Talose |
D-Psicose and L-Tagatose D-Fructose and L-Sorbose D-Allose and L-Talose D-Altrose and L-Galactose D-Glucose and L-Idose D-Mannose and L-Gulose |
Looking at Figure 8c: one can see that D-Lyxose, a C2 epimer of D-Xylose, will have C2-OH at axial position and D-Ribose, a C3 epimer of D-Xylose, will have C3-OH at axial position. D-Arabinose differs both at C2 and at C3 from D-Xylose, so it will have both C2-OH and C3-OH at axial positions. Similarly looking at Figure 8a and 8b, D-Allose, a C3 epimer of D-Glucose and connected by a double-yellow line, has its C3-OH at axial position. D-Tagatose and D-Psicose (Figure 8d) differ at C3 and C4, respectively, from D-Sorbose and hence will have their C3-OH and C4-OH, respectively, at an axial position.
In Summary, a way of determining the stereochemical relationships of D and L monosaccharides is proposed based on the simple and the generalized monosaccharide cycles, which show the 'cyclic' relationship of the monosaccharides. The above rules and illustrations would be useful tools in organic and biochemistry courses.
References.
[1] T. W. Rademacher, R. B. Parekh, & R. A. Dweck (2000) Ann. Rev. Biochem. 57, 785-838.
[2] M. L. Phillips, E. Nudelman, F.C.A. Gaeta, M. Perez, A.K. Shingel, S. Hakamori, & J.C. Paulson (1990) Science 250, 1130-1132.
[3] See Supplemental Information.
[4] K.P.C. Vollhardt, N.E. Schore (2003) Organic Chemistry: Structure and Function, 4 th edition, W.H. Freeman and Company, New York.
[5] (a) H. S. El Khadem (1988) Carbohydrate Chemistry: Monosaccharides and Their Oligomers, Academic Press: New York. (b) D. Horton, W. Pigman, Eds. (1972) The Carbohydrates, Chemistry and Biochemistry, 2nd ed., Academic Press: New York.
[6] A. D. Mcnaught (1997) Carbohydr. Res. 197, 1-92.
[7] BIO2010: Transforming Undergraduate Education for Future Research Biologists (2003), Board on Life Sciences: http://www.nap.edu/books/0309085357/html/
Instructions on how to draw the monosaccharide cycle (MoCycle) for aldoses (Figure 1, G = Glyceraldehyde, E = Erythrose, and T = Threose ):
1. Draw a dashed-magenta horizontal line ( ~1 cm long) and write 1, E to the left and T,2 to the right of the line. (Write a small size G above the line). [You may use my mnemonic “ Green Extra-Terrestrial Right Arm, eXtra Large!“ to remember the names and orders of aldoses having five carbons or less. This corresponds to Glyceraldehyde, Erythrose, Threose, Ribose, Arabinose, Xylose, and Lyxose.]
2. Draw four points (corners of a square) encircling the line and label them as 1, 2, 3, and 4 (left bottom corner as 1, for consistency purpose only).
3. Label the four points (1-4) as Ribose, Arabinose, Xylose, and Lyxose, respectively.
4. Connect the points by drawing line(s) and color them accordingly. Each point (corner) will have not more than one of each color of line.
a. 1-2, 3-4 (consecutive, add 1): Single-red line
b. 1-3, 2-4 (Jumps by one, add 2): Double-yellow line
c. 1-4, 2-3 (their sum is 5 i.e. (min + max)): Dashed-magenta line
5. Draw eight points (corners of a cyclooctane) encircling the square and number them as 1-8 (left bottom corner as 1, for consistency purpose only).
6. Write the name of the eight aldohexoses on corners 1-8, in the order Allose, Altrose, Glucose, Mannose, Gulose, Idose, Galactose, and Talose, respectively. [You may use the mnemonic popularized by Louis and Mary Fieser of Harvard University: “ All altruists gladly make gum in gallon tanks” to help you remember the names and orders of the aldohexoses.]
7. Connect the points by drawing line(s) and color them accordingly
a. 1-2, 3-4, 5-6, and 7-8 (consecutive, add 1): Single-red line
b. 1-3, 2-4, 3-5, and 6-8 (Jumps one, add 2): Double-yellow line
c. 1-5, 2-6, 3-7, and 4-8 (jumps three, add 4): Triple-green line
d. 1-8, 2-7, 3-6, and 4-5 (their sum is 9 i.e. (min + max)): Dashed-magenta line
Similarly, using steps 1-4 from the previous procedure, one can draw the MoCycle for ketoses (Figure 2). To remember the names and order of the ketoses of six carbons or less, you may use my mnemonic “ Eating Ribs, Xena Precisely Folds Silver Tags” which corresponds to Erythrulose, Ribulose, Xylulose, Psicose, Fructose, Sorbose, and Tagatose, respectively. Notice that carbon-2 of the ketoses is not a stereocenter so there will not be a single-red line for ketoses.
Instructions on how to draw the generalized monosaccharide cycle (MoCycle) for ketoses (Figure 3, E = Erythrose):
1. Draw a dashed-magenta horizontal line ( ~1 cm long) and write 1,D- E to the left and L-E,1’ to the right of the line. [You may use my mnemonic “ Eating Ribs, Xena Precisely Folds Silver Tags” which corresponds to Erythrulose, Ribulose, Xylulose, Psicose, Fructose, Sorbose, and Tagatose, respectively to remember the names and order of ketoses of six carbons or less].
2. Draw four points (corners of a square) encircling the line and label them as 1, 2, 2’, and 1’ (left bottom corner as 1, for consistency purpose only).
3. Label the four points (1, 2, 2’ and 1’) as D-Ribulose, D-Xylulose, L-Xylulose, and L-Ribulose, respectively.
4. Connect the points by drawing line(s) and color them accordingly. Each point (corner) will have not more than one of each type of line.
a. 1-2, 2’-1’ (consecutive, add 1): Double-yellow line.
b. 1-2’, 2-1’ (Jumps by one): Dashed-magenta line.
5. Draw eight points (corners of a cyclooctane) encircling the square and number them as 1-4 and 4’-1’ (left bottom corner as 1, for consistency purpose only).
6. Write the names of the four D-ketohexoses on corners 1-4, in the order D-Psicose, D-Fructose, D-Sorbose, and D-Tagatose, respectively and the names of the four L-ketohexoses on corners 1-4, in the order L-Psicose, L -Fructose, L -Sorbose, and L -Tagatose, respectively.
7. Connect the points by drawing line(s) and color them accordingly.
a. 1-2, 3-4, 4’-3’, and 2’-1’ (consecutive, add 1): Double-yellow line.
b. 1-3, 2-4, 4’-2’, and 3’-1’ (Jumps one, add 2): Triple-green line.
c. 1-4, 2-3, 3’-2’, and 4’-1’ (their sum is 5 i.e. (min + max)): Dashed-magenta line.
d. 1-4’, 2-3’, 3-2’, and 4-1’ (jumps three): Wiggly-blue line.
Similarly, using the seven steps from the previous procedure, one can draw the generalized MoCycle for aldoses of five carbons or less (Figure 4). Since carbon-2 of the aldoses is a stereocenter there will be a single-red line for aldoses. The generalized MoCycle for D- and L-aldohexoses can also be drawn with a similar procedure using the cyclohexadecane cycle (Figures 5). The generalized MoCycles show the epimeric relationships between the D and D, D and L, and L and L monosaccharides.
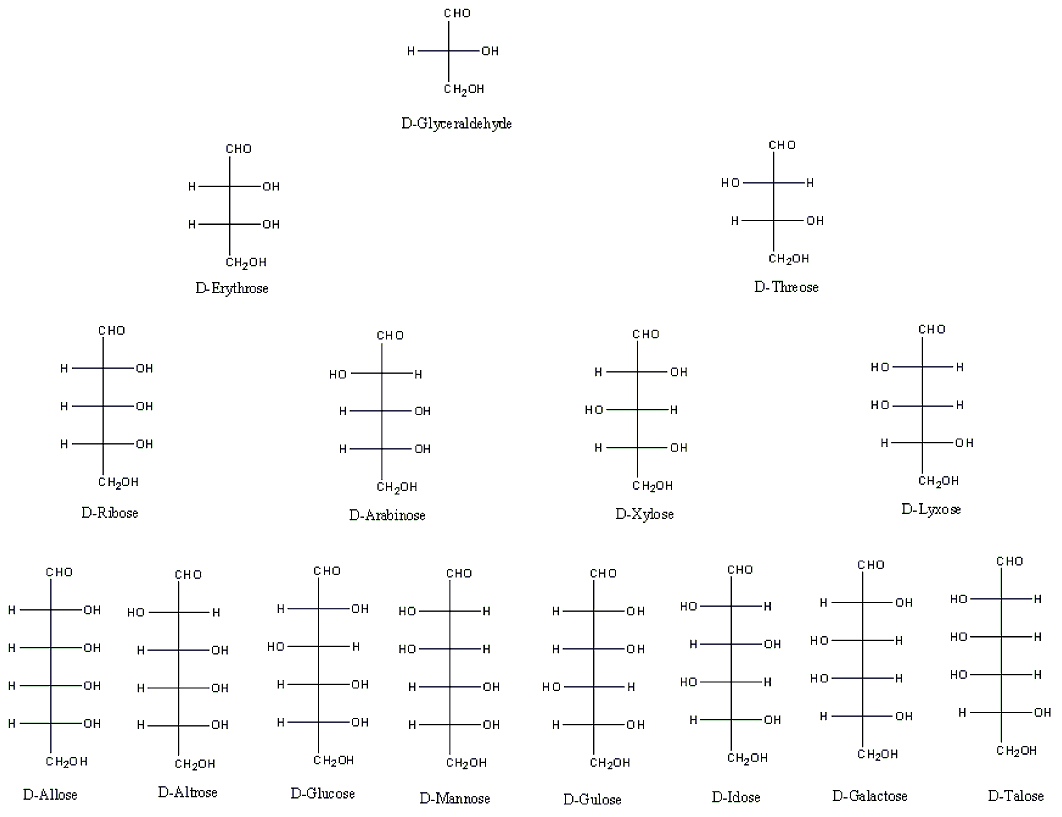
Figure 6 . Chart representation of the aldoses.
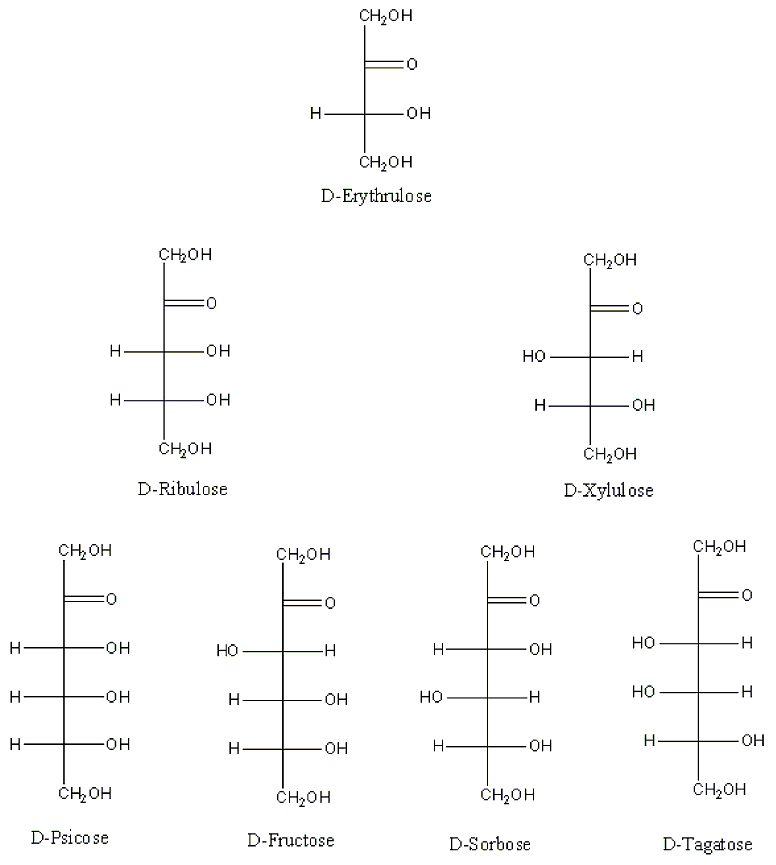
Figure 7 . Chart representation of the ketoses.
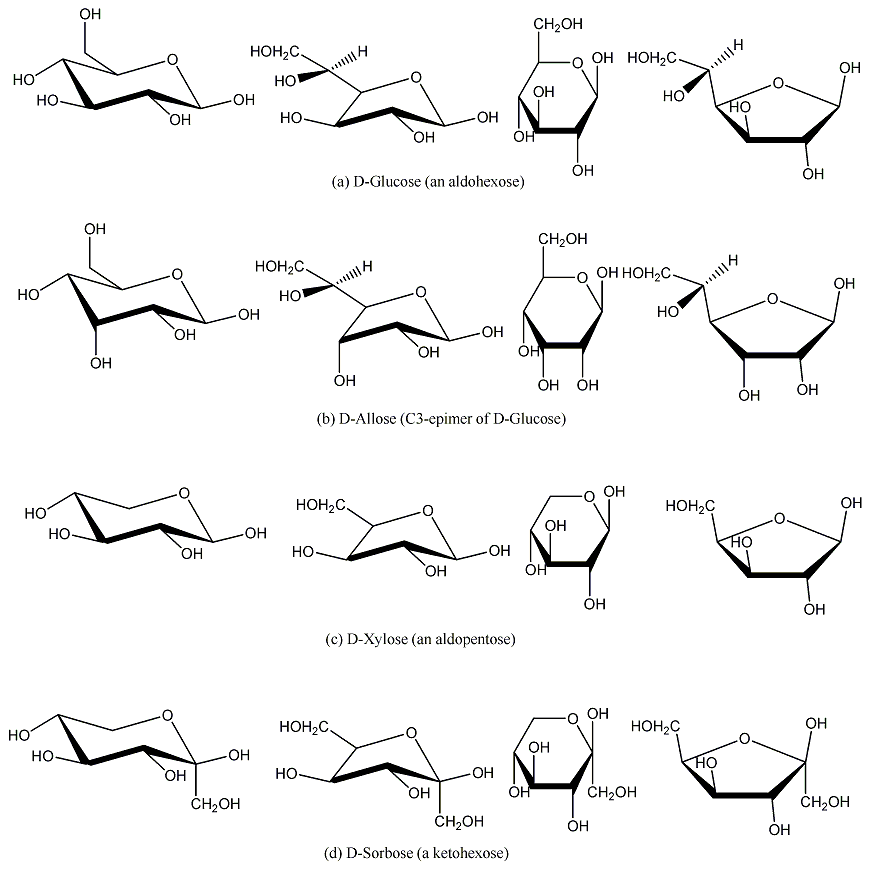
Figure 8 . Pyranose and furanose structures of D-Glucose, D-Allose, D-Xylose and D-Sorbose.
 Top
Top
