E-mail: ozden
Chemical Education Journal (CEJ),
Vol. 9, No. 2 /Registration No. 9-15 /Received July 3, 2006.
URL = http://www.juen.ac.jp/scien/cssj/cejrnlE.html
E-mail: ozden![]() gantep.edu.tr ,
mozden
gantep.edu.tr ,
mozden![]() hotmail.com
hotmail.com
Abstract
Drama and modeling games can be a very influential teaching strategy for meaningful learning in science education. In this drama, the students in the class role play actively for modeling atoms and formation of chemical bond concept. The students have a specific role in the drama. They learn the chemical bond concepts while enjoying and gaming. They all play the role and contribute to the process of learning.
They all have responsibility for modeling and playing in the drama. The students will not memorize science concepts in this model of learning but remember everything in the drama in detail, because this model of game or drama is his / her own production. The ionic and covalent bond concept is a basic concept that should be learned in elementary schools. In this drama, the students act as protons, electrons and neutrons in different atoms making ionic and covalent bond with each other. They move, rotate or become stagnant according to their roles in drama. They all speak together and show the harmony of atom model and formation of a chemical bond.
Key Words: Drama and game modeling, science
education, chemical bond
The main aim of primary science education should be to help children make meaningful sense of the world around them. This will enable them to develop into scientifically literate adults who have a critical understanding of the ideas of science, but who also have respect, care and sensitivity for the world they live in and their impact on it. If that goal is held central to teaching, then motivation, enjoyment and a sense of fun in learning become integral to planning, as meaningful learning only occurs when children engage fully in the process. Drama is an excellent medium for harnessing these features of learning, because it can simulate real-life experience and address issues in a way that may not be possible with other approaches to learning (Littledyke, M., 2004).
Many efforts have been made to improve the public understanding of science. The reason for lack of success in science lessons is the science educators and the learners having no motivation and enjoyment (Kara, A., Ozden, M.,2005).
Drama can be an effective tool to communicate, teach, learn
and assess science and technology. Drama is very popular with
pupils and can be used to stimulate discussion and debate. These
activities can be developed as far as you like, including ideas
for short role-plays in science classes right through to bringing
in the drama department and putting on a full production for the
school.
2. Drama for Educational Purposes
The usage of drama for educational purposes has been promoted by the science educator Joan Solomon (1989). She has worked on preparing scripts of dramas for student's role playing. First project of this science educator was "Galileo's Trial". Using drama to portray the lives of scientists may help students achieve profound understanding of the process of science and its nature if they are required to research, write, edit, perform and reflect on performances (Beichner,1994).
Using drama may allow students to get a real sense of science.
By taking a look at scientists' lives and playing their roles,
students may come to realize the absence of the so called science
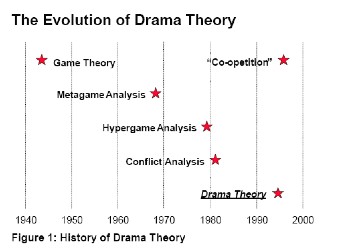
and the scientists' objectivity (McComas,1996). The evaluation of drama theory
is concluded in Figure
1.
Richard Ponting (1978) has produced student-initiated drama based on Durrenmatt's "The Physicists" and other plays in high school.
3. The Worth of Drama in Science
Drama can be a very influential instruction approach for enhancing meaningful learning in science. Active, participatory learning that draws directly on children's resources as social beings is central to drama. This provides strong motivation for learning and is particularly useful to help develop skills in communication and collaboration and in expressing ideas, values and opinions. Many of the ideas presented here are most appropriate for key stage 2 (ages 7-11), but there is also rich opportunity for development of key stage 1 (ages 5-7) and foundation stage (ages 4-5) drama and science through role-play in topics such as materials in the home or building site, health and the dentist or doctor, and so on. Many books and stories lend themselves to dramatization to develop scientific ideas. For example, ideas about forces can be developed through dramatization of MrGumpy's Outing or nursery rhymes such as Jack and Jill and The GrandOld Duke of York. Whole environments, such as the sea, a coal mine or stone age cave, canal, so be simulated in the classroom to provide a medium for dramatizing and learning about science (Littledyke, M., 1998).
The 5 steps for experience of learning through drama is shown in Figure 2. According to this figure, learning through drama can maintain the existential learning .
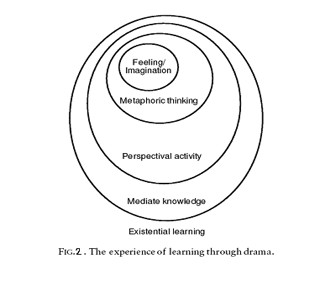
Although drama can take many forms, two are particularly relevant to providing a focus for reporting science: scientific models involving pupils; and real-life simulations that provide a medium for presenting scientific ideas and their social implications (Littledyke, M., Ross, K., Lakin, L., 2000).
4. Scientific Models in Drama and Game
Drama and game modeling can be used to reproduce science concepts through the teacher's instruction, or children can be challenged to create their own dramatized models to show their understanding.
Some common examples of typical games in the science curriculum are shown in Table 1.
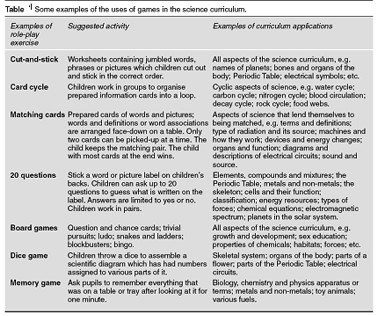
It is likely that this will involve some form of parody, but an associating narrative by the children explaining what the model represents enables them to show just how well they understand it. The chemical bond has become one of the most attractive subjects for drama. The purpose of this drama type of modeling in science education is to learn scientific concepts and enjoying them.
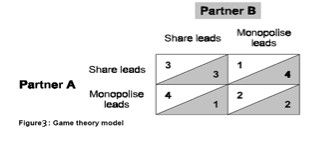
Figure 3 explains the game theory model in a different way from drama theory model. This figure helps to understand the difference between game and drama theory model.
Dramas may also be categorized according to whether they are presentational or experiential (Schaffner, Little, Felton & Parsons, 1984). Figure 4 offers a representation of this grouping, drawing also on Szatkowski's idea of 'aesthetic doubling' (see Odegaard, 2003). The presentational dramas have a major emphasis on communicating something to others outside the drama (e.g., teacher, peers, or parents). When a small group of students dramatize a scientific concept (e.g., the 'meiosis ballet' below), the intention is often communication to others. The experiential dramas focus on attempting to live through some aspect of an experience and adopting a motivation, opinion or attitude (e.g., a role-play with role cards about ethical issues in biotechnology).

The nature of drama is multi-faced, so reducing it to one content is not easy. When drama is depicted on a continuum from structured to explorative, it is implied that a structured drama/theatre most often is initiated and directed by the teacher (or actors) and is presentational (in the theatre there is an audience). Explorative drama is spontaneous, often student-driven, and experiential. In the following, I introduce examples of science and drama projects under the headings of each of the three science education perspectives. Under each heading, an attempt is made to present them in order of increased dramatic freedom. Figure 5 explains how drama may be used in science education as an overview.(Odegaard; 2003)
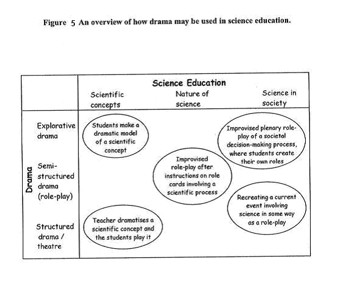
Dramatic activity may vary and take many different forms in the
classroom: in this section I identify some of the dimensions along
which it varies. The drama may be structured in a way where students
enact roles within the known framework of scientific theories:
for instance playing electrons in a circuit to illustrate the
scientific concept of electricity. The dramatic activity may be
impulsive, creating the moment, as it were; students have to improvise
who they are and what to say. At any point along this continuum
a drama can be more or less spontaneous. An intermediate form
could be an improvised role play with a structured frame (e.g.,
role cards that describe the participating roles). Another continuous
variable is the degree of teacher involvement: that is, whether
it is the teacher that impels the drama or the students. A group
of students who create their own model of a scientific concept
are together reconstructing knowledge so as to enhance their conceptual
understanding. In order to guide the students, it may sometimes
be necessary for the teacher to provide scaffolds in complicated
scientific matters. A similar four-way continuum is offered by
Brown & Pleydell (1999),
and a re-worked version of there approach is presented in Figure 6.(Odegaard; 2003)
One type of chemical bonding is ionic bonding. In such a case, one atom will give up one or more valance electrons to the other atom. The atom losing electrons becomes a positive (+) ion and the one gaining electrons becomes a negative (-) ion. The electrical force keeps the atoms close together and bonds them into a molecule.
Salt or Sodium Chloride (NaCl) is a good example of an ionic bonding (Figure 7). Sodium (Na) has 1 valance electron and Chlorine (Cl) has 7 electrons in its outer orbit. If Sodium lost its valance electron, its next shell will be full. But that would also make Sodium a positive ion. If Chlorine gained 1 valance electron, its shell would be full with a maximum of 8 electrons, and it would then be a negative ion.Thus Sodium Chloride (NaCl) is a bonding of the Na+ ion and the Cl- ion.
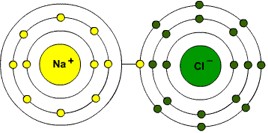
In its solid form as table salt, the Na+ and the Cl- ions are held in place in a crystalline lattice. When dissolved in water, the ions freely roam about the solution. Note that the combination of these two elements can result in a violent reaction, giving off heat and perhaps even an explosion. Seldom is Na directly combined with Cl to form NaCl. Usually the combination is done indirectly with other compounds or in a water solution. But the fact that the bonding process gives off energy means that the molecule is fairly stable and not easy to separate.
Most common type of chemical bonding is single covalent bonding, where one pair of valence electrons is shared by the two atoms. Valence electrons are those that are in the outer orbit or shell of an atom.
A good example of single covalent bonding is the Hydrogen molecule (H2 , Figure 8).
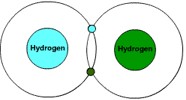
Since the atoms are sharing the other's electron, both appear to have the first orbit or shell filled with the maximum of two electrons.
Another example of single covalent bonding is the water (H2O) molecule. Adding hydrogen gas molecules (H2) to oxygen gas molecules (O2) can result in an explosion if lit by a flame or spark. The end product is the very stable water molecule (H2O). The chemical equation is:
The resulting molecule has single covalent bonding (Figure 9):

You can see that with the sharing of electrons, each Hydrogen atom has two valence electrons, thus filling their outer orbits. Likewise, Oxygen now has 8 outer orbit electrons. This makes for a good chemical bond and a stable molecule. Usually, electrolysis is required to separate the hydrogen and oxygen from water.
8. Dramatization and Game Model for Ionic Bonding
All of the dramatization should be realized in a large area like a school garden or sports area. First of all, the bonding concept should be dramatized. Two students (boy and girl) from the class take each other by the hand and say we formed a chemical bond together loudly. This model represents the chemical bonding. After this modeling, the ionic bonding concept should be dramatized. For example, in this dramatization, Lithium (Li) and Hydrogen (H) atoms can be represented by the students. Since Li has 3 protons, 4 neutrons in nucleus and 3 electrons in the orbits , we need 10 students from the class. 3 male students modeling protons and 4 female students modeling neutrons must sit down in the centre as an dense society and be stagnant position. They shouldn't move during the dramatization. After, placing nucleus of atom, now we call 3 female students from the class and two of them rotate slowly around the nucleus, the other one rotates slowly around two students near to nucleus. They should be careful about preventing collisions. At the other corner of the school garden, the H atom modeling should be settled. H atom consists of 1 proton, zero neutron and 1 electron. For this modeling, again, 1 male student modeling proton and 1 female students modeling electron can be placed like the other atom model. In this atom modeling, male student should be stagnant and the female one should rotate slowly around the male student.
After each atomic model is settled, the structure of ionic bonding is explained as follows:
Ionic bonding is bonding that metal atom (Li) gives one electron to nonmetal atom (H) and nonmetal atom takes this electron for being stable. When Li metal atom gives an electron, it becomes +1 positive cation; all of the students in the Li group make vocal and say we all became +1 cation and then H takes this electron and becomes -1 anion; in the same way, all of the students say became anion . The positive ions attracts the negative ions. To apply this electron trading, one of the student modeling an electron in the outer orbit of Li atom slowly comes toward H atom modeling and then begin rotating around the H atom nucleus with the only electron outside the nucleus of H atom. Since this action makes atoms positive and negative ions and attract each other, two atoms at the opposite corner of the garden, start to come close each other and making a compound named LiH (lithium hydride). The two groups at the corner of the garden, rapidly come close to each other and become a compound saying we are lithium hydride compound all together. Thus, dramatization of ionic bonding is completed with the contribution of 12 students.
9. Dramatization and Game Modeling for Covalent Bonding
In this dramatization, Hydrogen and Fluorine atoms can be represented by the students. Since Hydrogen atom has 1 proton and 1 electron, we need one boy and one girl student from the class. For this model, 1 male student modeling proton at the center in the nucleus being stagnant and 1 female student modeling electron can rotate around the nucleus slowly. At the other corner of the school garden, the Fluorine atom modeling should be placed. Since Fluorine atom consists of 9 protons, 10 neutrons and 9 electrons in the orbits, we need 27 students from the class. 9 male students modeling protons and 10 female students modeling neutrons must sit down in the centre and be in stagnant position. After, placing the nucleus of Fluorine atom, we can call 9 female students; 2 of them rotating slowly near the nucleus in the first orbit and rest of them (7 students) rotating slowly near the first orbit in the second (last) orbit. The students modeling electrons, should not be collide during rotation. After these two atom models placed, the structure of covalent bonding is explained as follows:
Covalent bonding is a bonding consisting of two nonmetal atoms sharing each other's electrons. The Hydrogen and Fluorine atoms share their one electron in the outer orbit of the shell. Thus, each atom's nucleus attracts these shared electrons and the covalent bonding between two atoms is being formed. To apply this electron sharing, one student from each atom rotating at the outer orbit, comes near to each other and takes each other by the hand. So, when the covalent bonding is starting to form, the two groups with all population come slowly to each other and say we formed a covalent compound called Hydrogen Fluoride together. So, the dramatization of covalent bonding is completed by the instructor and the students.
Dramatic play can be a versatile tool for enhancing children's learning in different subject areas of learning. Besides its potential as a learning tool, dramatic play offers teachers a unique opportunity to learn more about their students. With this knowledge teachers can design educational experiences relevant to their students' needs. Understanding the different levels of interaction in play helps teachers to know when to encourage students with more or less leadership ability and when to intervene when some students try to dominate the development of the play. The emergence of conflicts should not be avoided or perceived as negative. Instead, teachers should view conflicts as opportunities for students to rehearse their ability to negotiate ideas. Teachers can serve as mediators rather than impose their authority on students to solve a problems on those occasions when students are unable to reach a solution by themselves (Sierra, 1998). The role of teachers during the dramatic play experience is of vital importance. They need to be prepared to raise questions and to challenge assumptions when oppressive relations or cultural stereotypes emerge in the students' play stories. For example, they can offer much needed guidance when children attempt to solve problems by violent means or by imposing gender or racial prejudices. As teachers use dramatic play in their classrooms, they should remain sensitive to the quality of the relationship they establish with the children. The responsibility to create a comfortable environment wherein children can engage in dramatic play rests with the teacher. That is, the dramatic play facilitators or teachers need to be sensitive to children's creations; to be imaginative and creative in suggesting proposals for dramatic play; to be ready to discern and assess differences in individual and group behaviors; and to have the ability to interpret the content that emerges from the play-stories. Training in creative dramatics and children's theatre should include strategies to improve teachers' sensibility and competencies needed to interpret dramatic play (Sierra, 1998).
The students in the game age, learn the concepts easily with game modeling and drama. The selection of instruments and area is very important for these activities. The rule and the process of game or drama should be clear and explained carefully in details. The game modeling or drama should be consistent with the learning area and concept. If this consistency can be provided, then the lessons will be instructive, interesting and enjoyable. The game modeling or drama for science teaching should be as follows:
Andrew, T. (1998), "Drama Without Tears, Strategic Leadership Sciences - Europe", SAIC, 1 Northumberland Avenue, London.
Beichner, R. (1994), "Multimedia editing to promote science learning", Journal of Computers in Mathematics and Science Teaching, 13, 147-162.
Brown, V., Pleydell, S. (1999), "The dramatic difference. Drama in preschool and kindergarten classroom", Portsmouth, NH, Heinemann.
Kara, A., Ozden, M. (2005), "Secondary Students' Attitudes towards Chemistry Lesson", XIV. National Educational Sciences Congress, Pamukkale University, Education Faculty, Denizli, Turkey.
Littledyke, M. (1998), "Live issues: drama strategies for personal social and moral education", Birmingham, Questions Publishing Company.
Littledyke, M., Ross, K., Lakin, L. (2000), "Science knowledge and the environment: a guide for students and teachers in primary education", London, David Fulton.
Littledyke, M. (Sept/Oct 2004), Primary Science Review, 84.
McComas, W. (1996), "Ten myths of science: Reexamining what we think we know about the nature of science", School Science and Mathematics, 96, 10-15.
Odegaard, M. (2003), "Dramatic science: A critical review of drama in science education", Studies in Science Education, 39, 75.
Ponting, R. L. (1978), "Combining Physics and Drama", The Physics Teacher, 16 (7), 482-483.
Schaffner, M., Little, G., Felton, H., and Parsons, B. (1984), "Drama, language and learning", Reports of the drama and Language research Project. Speech and drama center, Education Department of Tasmania, NADIE Papers No.1, Tasmania: National Association for Drama in education.
Szatkowski, J. (1985), "Nar kunst kan brukes", in J. Szatkowski and C. B. M. Jensen (Eds.), "Dramapedagokikk II i Nordisk perspektiv" (pp. 136-182), Grasten, Denmark, Teater-forlaget Drama.
Sierra, Z. (1998), "Play for Real: Understanding Middle School Children's Dramatic Play", Unpublished Doctoral Dissertation, University of Georgia, Athens, GA.
Solomon, J. (1989), "The Retrial of Galileo", in Don Emil Herget (ed.), "The History and Philosophy of Science in Science Teaching", Florida State University, Tallahassee, pp. 332-339.
CEJ Vol. _, No. _, Contents