Florida Atlantic University, College of Education
2912 College Avenue, Davie, Florida 33314, USA
E-mail: david@fau.edu
A computer program (HyperChemistry) developed for analyzing chemistry problem solving protocols is revisited and re-examined. A secondary analysis of protocol data from high school students solving a molarity problem using the program is reported. Limitations are discussed.
This paper revisits the Hyperchemistry computer program developed for studying chemistry problem solving protocols, and reevaluates the original data (Kumar and Helgeson, 1996), for any generalizable solution profile. A note should be made that the aim of the Kumar and Helgeson (1996) study was to investigate the effect of two program versions (Macintosh Powerbook and Pen-Point) on chemistry problem solving among different ethnic groups.
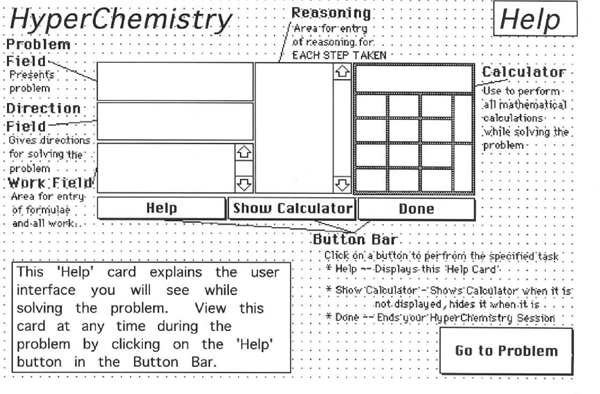
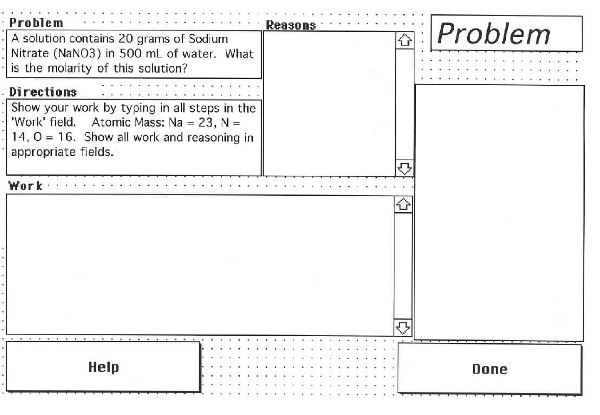
Once HyperChemistry is loaded on a Macintosh Powerbook computer, the screen is partitioned into a Work Field and a Reasoning Field. See Appendix 1. These fields are text fields. The problem solver will enter the step-by-step solution in the Work Field and enter the reason for each step in the Reasoning Field. HyperChemistry is programmed to keep track of all entries made inside these two fields and the amount of time spent in each field, from entering the field to exiting. The computer mouse is used to move the cursor from one field to the other. The software includes a help card. See Appendix 2.
The chemistry problem solved using HyperChemistry follows:
"A solution contains 20 grams of Sodium Nitrate (NaNO3) in 500 ml of water. Calculate the molarity of the solution. Given atomic mass of Na = 23; N = 14; O = 16."
The software recorded solution protocols in Simple Text under the sign in name of each student. Figure 1 shows a sample printout of a solution protocol in terms of the Protocol by Time and the Development of Solution to the Problem, and the Protocol Summary. The Protocol by Time and the Development of Solution to the Problem is the time and number of times one entered Work Field and Reasoning Field and the respective entries made in each field. The Protocol Summary is the summary of activities in the Work Field and Reasoning Fields.
FIGURE 1.
A Sample Problem Solving Protocol Recorded by HyperChemistry
___________________________________________________
Event Log ---->HyperChemistry
Student Id =77777
File created at 10:25:48 AM on Friday, Monday 21, 2001
ok On Title Screen Clicked 10:25:48 AM
begin Problem Button Clicked 10:26:57 AM
[PROTOCOL BY TIME and DEVELOPMENT OF SOLUTION]
mouse Entered Work Field 10:27:52 AM
WORK FIELD AT: 10:31:00 AM
20g/.5Lx1/23+14+16(3)
END WORK FIELD AT: 10:31:00 AM
mouse Entered Reasoning Field 10:31:01 AM
REASONING FIELD AT: 10:32:35 AM
Change grams to moles, and divide the moles by the liters of solution, and that
END REASONING FIELD AT: 10:32:35 AM
mouse Entered Work Field 10:32:54 AM
WORK FIELD AT: 10:32:54 AM
20g/.5Lx1/23+14+16(3)
END WORK FIELD AT: 10:32:54 AM
mouse Entered Work Field 10:32:55 AM
WORK FIELD AT: 10:34:19 AM
20g/.5Lx1/23+14+16(3)
40x1/85
.4705882 M
END WORK FIELD AT: 10:34:19 AM
mouse Entered Work Field 10:34:19 AM
WORK FIELD AT: 10:35:01 AM
20g/.5Lx1/23+14+16(3)
40x1/85
.4705882 M
4.7x10(1-)
END WORK FIELD AT: 10:35:01 AM
done Button Clicked 10:35:06 AM
[PROTOCOL SUMMARY]
WORK FIELD -------------
20g/.5Lx1/23+14+16(3)
40x1/85
.4705882 M
4.7x10(1-)
END WORK FIELD ---------
REASONING FIELD --------
Change grams to moles, and divide the moles by the liters of solution, and that will give you the molarity.
END REASONING FIELD ----
Student Id = 00107
Done at 10:35:06 AM
___________________________________________________
A secondary analysis of the solution profiles from the Kumar and Helgeson (1996) study (involving 60 high school chemistry students) revealed the following steps in solving the molarity problem above.
1. The molar mass of sodium nitrate was calculated.
2. The number of moles was calculated by dividing the mass of sodium nitrate by molar mass.
3. The amount of solvent was converted from milliliters to liters.
4. The number of moles of sodium nitrate was divided by the volume of solvent to determine the molarity.
It should be noted that this profile concurs with the one in Heyworth (1989) done independently using traditional paper and pen method and follow up interviews.
Additional applied research is needed before establishing HyperChemistry as a suitable tool for analyzing protocols in chemistry problem solving. One of the disadvantages of the software is "noise" due to programming error. The software records every time the mouse enters and leaves the Work and/or Reasoning Fields. The HyperChemistry program needs to undergo considerable revision in order to make it suitable for problem solving research.
REFERENCES
The screen layout of the HyperChemistry . (A schematic version appeared in Kumar, D. D. , & Helgeson, S. L. (1996). Effect of computer interfaces on chemistry problem solving among various ethnic groups: A comparison of Pen-Point and Powerbook computers. Journal of Science Education and Technology, 5(2), 121-130. Used with permission.)

A layout of the HyperChemistry help screen. (A schematic version appeared in Kumar, D. D. , & Helgeson, S. L. (1996). Effect of computer interfaces on chemistry problem solving among various ethnic groups: A comparison of Pen-Point and Powerbook computers. Journal of Science Education and Technology, 5(2), 121-130. Used with permission.)